Warehouse Mapping Done Right: Qualification & Mapping of Storage Rooms
Enhance your warehouse operations and improve inventory tracking and layout efficiency with this guide to effective mapping.
Learn how GxP qualification and mapping secure product safety and compliance in pharmaceutical manufacturing and supply chain management.
Ever wondered what a validation, qualification, or temperature mapping means and how it is applied in the GxP-regulated environment? This article answers these questions while explaining how each process supports regulatory compliance, product quality, and patient safety. You’ll also learn why a risk-based approach is essential in achieving GxP validation and compliance with Good Manufacturing Practice (GMP), Good Laboratory Practice (GLP), and Good Clinical Practice (GCP).
There are many GxP guidelines for good business practices, and even when the guidance is contradictory, all sources agree on one thing: if you work in the pharmaceutical or medical device sector, you must comply with Good Manufacturing Practice (GMP) and Good Distribution Practice (GDP). You must produce, handle, store, and transport your temperature-sensitive products in qualified facilities with validated equipment, using compliant processes that meet regulatory requirements. This guide explains everything you need to know about the validation process, qualification, and mapping—critical parts of any quality management system (QMS).
According to GxP regulations such as GMP, GDP, and Good Laboratory Practice, validation is a documented and mandated process that guarantees the anticipated results of a manufacturer. All functions relevant to product quality, data integrity, and regulatory compliance must be validated.
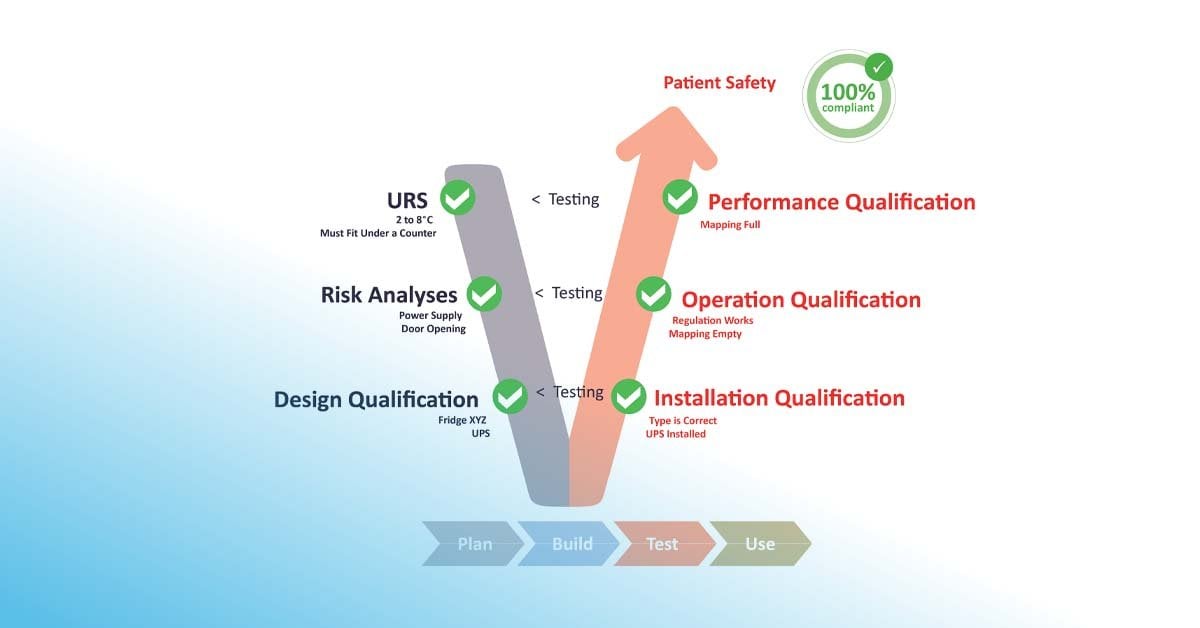
Qualification is the process of proving that facilities and equipment work properly to deliver quality results, which fulfil the intended purpose in accordance to the user requirement specification (URS). Apart from regulatory compliance and stipulation, qualification is paramount to ensuring the preservation of product quality, continuity of supply and a healthy, effective outcome for consumers and patients.
Qualification is the process of proving that facilities and equipment work properly to deliver quality results, which fulfil the intended purpose in accordance to the user requirement specification (URS). Apart from regulatory compliance and stipulation, qualification is paramount to ensuring the preservation of product quality, continuity of supply and a healthy, effective outcome for consumers and patients.
Beyond GxP compliance, qualification ensures continuity of supply, data integrity, and ultimately, patient safety.
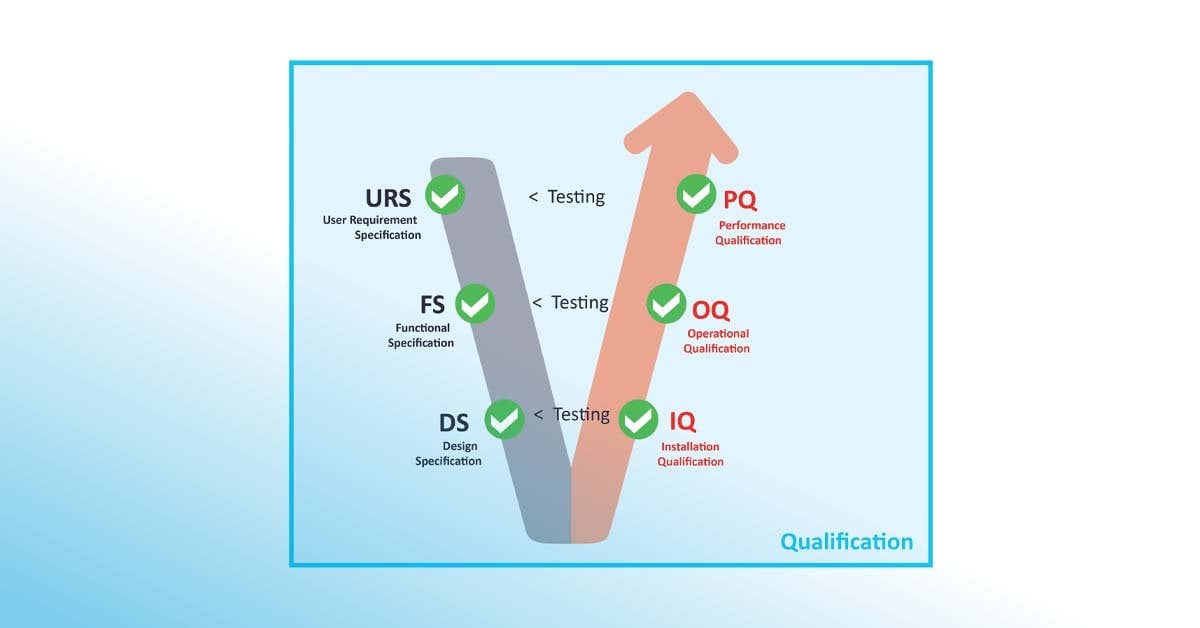
According to the GAMP® 5 V-shaped model framework, the qualification plan (QP) is developed to define the series of actions and analyses including functional specification (FS), design qualification (DQ), risk assessments, implementation, installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ). The result of the qualification process is captured, recorded, and approved within the qualification report (QR). A series of tests should be adapted at each stage to ensure that any issues identified are completely resolved.
An easy-to-follow guide to help readers understand GMP
It’s a scientific fact that cold air is denser and heavier and pushes the warmer, lighter air upward. Displacement and disparity of air represents an unknown risk to temperature sensitive products manufactured, stored, and transported in controlled environments, whether it be a warehouse, a container, a refrigerator, or a freezer. Temperature mapping is the process of identifying uncertainty and volatility to a known entity by measuring and documenting the temperature at multiple locations inside a chamber, otherwise known as thermal distribution. Temperature mapping identifies the areas that are within the safe parameters for storing your product.
Preparing for mapping and producing the analysis summary can be an involved and time-consuming process. The mapping plan and protocol incorporates the risk assessment to define and to rationalize the placement of temperature data logging devices. It’s important that the devices used are manufactured through validated processes. They should be calibrated at multiple points to cover the anticipated environmental range. The number of measurement points required are set out in accordance with volumetric calculations. A mapping study is conducted over a predefined period in accordance with regulatory guidance and globally recognized standards.
The mapping study observes the temperature in specific locations within the space to create a mapping grid. The mapping grid observes two principles:
According to the GAMP® 5 V-shaped model framework, the qualification plan (QP) defines the series of actions including Design Qualification (DQ), Installation Qualification (IQ), Operational Qualification (OQ), and Performance Qualification (PQ). These steps align with Computer System Validation (CSV) principles applied in GxP systems to ensure software development and hardware integration meet quality standards.
All results are captured within a Qualification Report (QR) that supports audit trails and demonstrates adherence to CFR Part 11 and GxP guidelines.
Warehouse Qualification: Ensure your storage and handling environments are compliant and follow a risk-based approach.
Equipment Qualification: Verify that all temperature monitoring devices, sensors, and data loggers are GxP-compliant, properly calibrated, and validated according to Good Laboratory Practice.
It’s a scientific fact that cold air is denser and heavier and pushes the warmer, lighter air upward. Displacement and disparity of air represents an unknown risk to temperature sensitive products manufactured, stored, and transported in controlled environments, whether it be a warehouse, a container, a refrigerator, or a freezer. Temperature mapping is the process of identifying uncertainty and volatility to a known entity by measuring and documenting the temperature at multiple locations inside a chamber, otherwise known as thermal distribution. Temperature mapping identifies the areas that are within the safe parameters for storing your product.
Preparing for mapping and producing the analysis summary can be an involved and time-consuming process. The mapping plan and protocol incorporates the risk assessment to define and to rationalize the placement of temperature data logging devices. It’s important that the devices used are manufactured through validated processes. They should be calibrated at multiple points to cover the anticipated environmental range. The number of measurement points required are set out in accordance with volumetric calculations. A mapping study is conducted over a predefined period in accordance with regulatory guidance and globally recognized standards.
The mapping study observes the temperature in specific locations within the space to create a mapping grid. The mapping grid observes two principles:
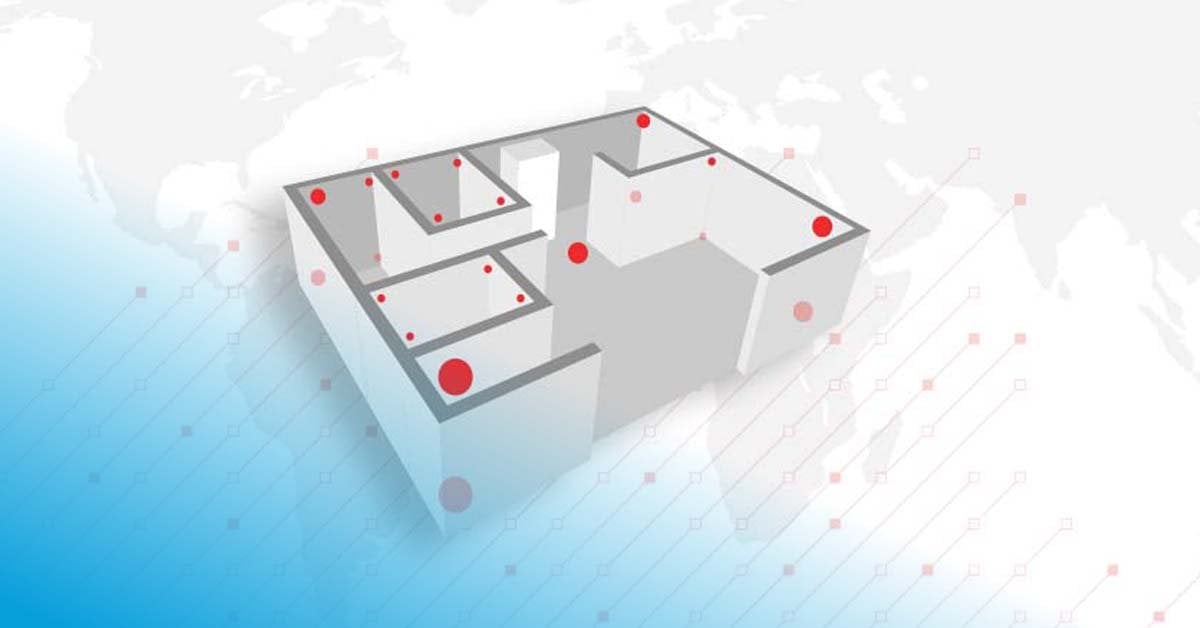
Mapping setup: Sensors are installed and configured, marked visibly, and placed at the appropriate mapping points.
The resulting purpose of the mapping analysis and summary report is to pass or fail equipment or areas based on determination of hot and cold spots, including consideration to the intended range and acceptance criteria. Average temperature conditions and sometimes mean kinetic temperatures (MKT) are also analyzed to identify less obvious risks or trends. These factors are captured in the summary report and should be accompanied with relevant recommendations and ongoing action plans, like where to place monitoring loggers within a chamber for continuous monitoring.
For temperature-controlled equipment and areas, mapping analysis forms part of the qualification plan. Often mapping is carried out during multiple stages of the qualification process— typically during IQ, OQ, and PQ phases. Mapping is the examination and evaluation part of qualification, providing you with the data for trends, simulated failures, and thermal reaction times to assess. Mapping during qualification often involves varied product loads inside the chamber under study, starting off empty and moving towards a typical operational load.
A mapping study defines the behavioral pattern within the room, while the qualification study follows through with a detailed analysis to show the design and operations match the expected results.
There are no exact formal requirements. So, whosoever wants to perform the process is free to choose what form and document structure to use. However, you should observe good documentation practices. Use clear document naming, versioning, and change the history and ownership of the document.
According to GxP validation guidelines, you must qualify all equipment impacting a patient's safety and authenticate its processes.
Some of the relevant equipment includes:
Qualifying temperature-controlled refrigerators involves risk-based testing for airflow, loading, and door openings. Mapping ensures regulatory compliance and ongoing data integrity.
Large storage facilities require seasonal temperature mapping due to ambient influences. Cold rooms often require less frequent mapping but still demand validation for GxP compliance.
Transport qualification ensures temperature-sensitive goods remain within range during transit. Both passive insulated boxes and active containers must be qualified and mapped to meet Good Distribution Practice and CFR Part 11 requirements.
In the pharmaceutical sector, the qualifying process differs depending on the equipment and space. In some instances, specialized engineering firms conduct the qualification. ELPRO uses the GAMP® 5 V model as a guide, the steps 3, 4, 5, and 6 being the qualification portion.
Here are some valuable examples of how to conduct qualification and temperature mapping in various circumstances:
Qualifying a refrigerator is straightforward because it is a freestanding gadget. Since these appliances operate in a room or environment with controlled temperature, risks are limited to air outlet blockage, power outage, improper loading, and door opening. In comparison to more intricate devices and personalized equipment, there are minimal risks.
Companies usually operate numerous refrigerators or freezers, so the qualifying processes are often done in the following steps:
Storage facilities, cold rooms, and warehouses are not the typical off-the-shelf refrigerators. Each product suits its industry through customized control systems, designs, and airflow. However, such facilities have many variations, each having different features and applications that directly affect the strategy choice.
Warehouse
Type: Large room/hall with open space or high racks
Typical Temperature Range: 2 °C to 8 °C or 15 °C to 25 °C.
Implications on Qualification & Mapping: Warehouses typically have outer walls directly exposed to ambient conditions and seasonal effects. Insulation of the building and the absence of windows and other openings are vital. Since there is influence from ambient conditions, winter and summer mappings are necessary. Same applies for the loading dock.
Cold Room
Type: Smaller room/hall with open space or racks.
Typical Temperature Range: 2 to 8 °C or -20 °C
Implications on Qualification & Mapping: Cold rooms are typically rooms inside a building and the walls have no direct exposure to ambient conditions and seasonal effects. Often there is no need to perform a separate winter and summer mapping.
Loading Docks and Other Facilities
Type: Small room with racks
Typical Temperature Range: 15 °C to 25 °C or 2 °C to 30 °C or <40 °C
Implications on Qualification & Mapping: The framework and complexity of the qualification and mapping of other facilities will be defined by the stored/handled products, their required label condition, and their length of stay within the facility.
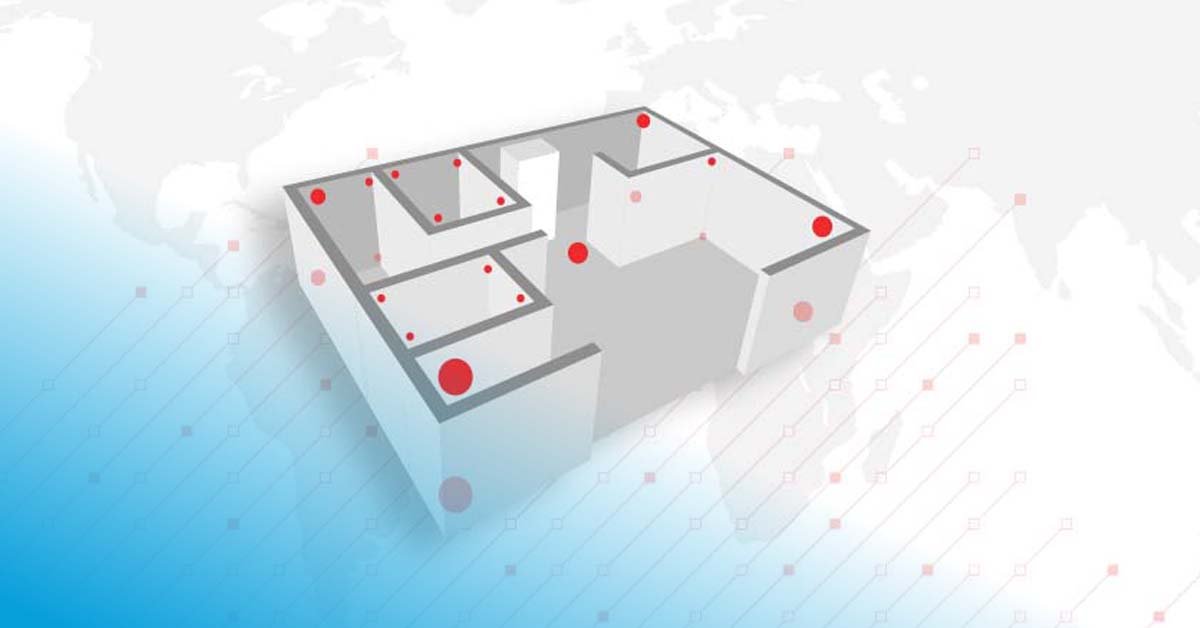
Since companies usually operate many trucks and vans as fleets, the qualifying processes are performed in fleets as follows:

Qualifying a transport box or container is not as easy since the object to be qualified moves around in an uncontrolled environment. First, clarify its purpose and shipment method, whether via road, air, or water. Take into consideration the transportation time and the temperature range at the beginning, during transit, and at the destination.
There are two ways to confirm the appropriate ambient temperatures during GxP validation:
There are differences between passive insulated boxes and active heating/cooling containers. Here is what you should know about the two:
High-level Risk Appraisal: The highest risk is a packaging error (e.g., wrong conditioning of PCM). As soon as a shipment is on its way, the solution is very stable and there are only minimal risks.
High-level Risk Appraisal: While active containers are easy to load, there are significant risks during shipment involving human error (e.g., no recharging) and mechanical failure. Heating and cooling containers consist of hundreds of mechanical and electronic components which have the potential to fail.
Once you choose the container and define its purpose, you can start the qualifying process by applying the V model steps, as explained earlier.
GxP validation of a transportation route or a network begins by qualifying all the equipment elements; you enable the location of the right subjects within a network. To find the proper subjects, you must cluster the equipment, such as boxes, fleets, and containers.
Clustering criteria of the transport elements include:
After clustering the network into similar categories, you can perform a high-level risk analysis. Cluster-specific risks should be described and evaluated. The higher the risk rating, the more focus must be given when defining mitigation measures. After performing the clustering and risk analysis, each “cluster element” must now be qualified. The following table shows an overview of potential clusters, risks, and mitigation strategies:
/Qualification%20and%20Mapping/Risk%20Strategy.png?width=855&name=Risk%20Strategy.png)
Now you know how to qualify, validate, and map various types of GxP systems and environments to comply with GxP regulations, CFR Part 11, and other regulatory requirements.
Maintaining data integrity, quality standards, and regulatory compliance is essential for pharmaceutical, medical device, and biotech companies. Partnering with ELPRO gives you access to expert GxP validation services, temperature mapping solutions, and environmental monitoring technologies — all built to ensure your operations remain GxP-compliant, audit-ready and efficient.
Contact ELPRO today to learn how our experts can help you achieve complete compliance and quality assurance across your GxP-related systems.
GxP stands for "Good x Practice," where x represents different fields such as Manufacturing (GMP), Laboratory (GLP), and Distribution (GDP). It is a general term for regulations and guidelines ensuring quality, safety, and compliance in industries like pharmaceuticals, biotechnology, and healthcare.
Some common GxP standards include:
These guidelines help maintain product quality, regulatory compliance, and patient safety.
The key difference between GxP (Good Practice) and non-GxP projects lies in the regulatory standards and compliance requirements that apply to them. These distinctions are especially relevant in industries such as pharmaceuticals, biotechnology, and healthcare, where product quality, patient safety, and regulatory compliance are of paramount importance.
GxP Projects
GxP refers to a set of regulatory guidelines aimed at ensuring products are consistently produced and controlled according to quality standards. These guidelines are essential in industries like pharmaceuticals, life sciences, and healthcare.
Non-GxP Projects
Non-GxP projects are those that do not require compliance with GxP regulations and may not be subject to the same stringent oversight. These projects typically focus on general operational improvements rather than compliance with industry-specific standards.
Key Differences
In summary, GxP projects are highly regulated and require stringent documentation and compliance measures, whereas non-GxP projects are generally more flexible and not bound by the same level of regulatory scrutiny.
GxP stands for “Good [X] Practice,” where the “X” can represent different areas such as manufacturing (GMP), laboratory (GLP), or clinical (GCP). It is a set of guidelines and quality standards used in the pharmaceutical, biotech, and life sciences industries to ensure that products are safe, effective, and consistently produced. Following GxP helps companies comply with regulations, maintain product quality, and protect patient safety.
GxP principles are applied in several regulated industries beyond pharmaceuticals, but the focus and requirements can differ:
Summary:
While GxP principles—like documentation, traceability, and quality control—are common across industries, pharma GxP is typically the most stringent because it directly impacts patient safety and product efficacy. Other industries may adopt GxP practices to protect consumers, but the regulatory oversight and risks differ.
Enhance your warehouse operations and improve inventory tracking and layout efficiency with this guide to effective mapping.
Quality control is a critical element in laboratory monitoring that helps ensure product integrity and process accuracy.
The basic requirements for accurate temperature monitoring.