Direct-to-Patient Shipment
Monitoring Solutions for Direct-to-Patient Logistics
Ensuring product integrity during temperature-controlled direct-to-patient (DtP) deliveries is critical. ELPRO provides end-to-end environmental monitoring solutions that offer complete visibility from packaging through to patient receipt—helping safeguard product quality and patient safety at every step.
- Kit-level temperature monitoring from packaging through last-mile delivery
- Validated single-use USB PDF data loggers and smart indicators for clear “OK”/“Not OK” status
- Remaining stability budget calculation, transparent compliance documentation and excursion visibility
- Fully GxP-compliant solutions supporting GMP and GCP requirements
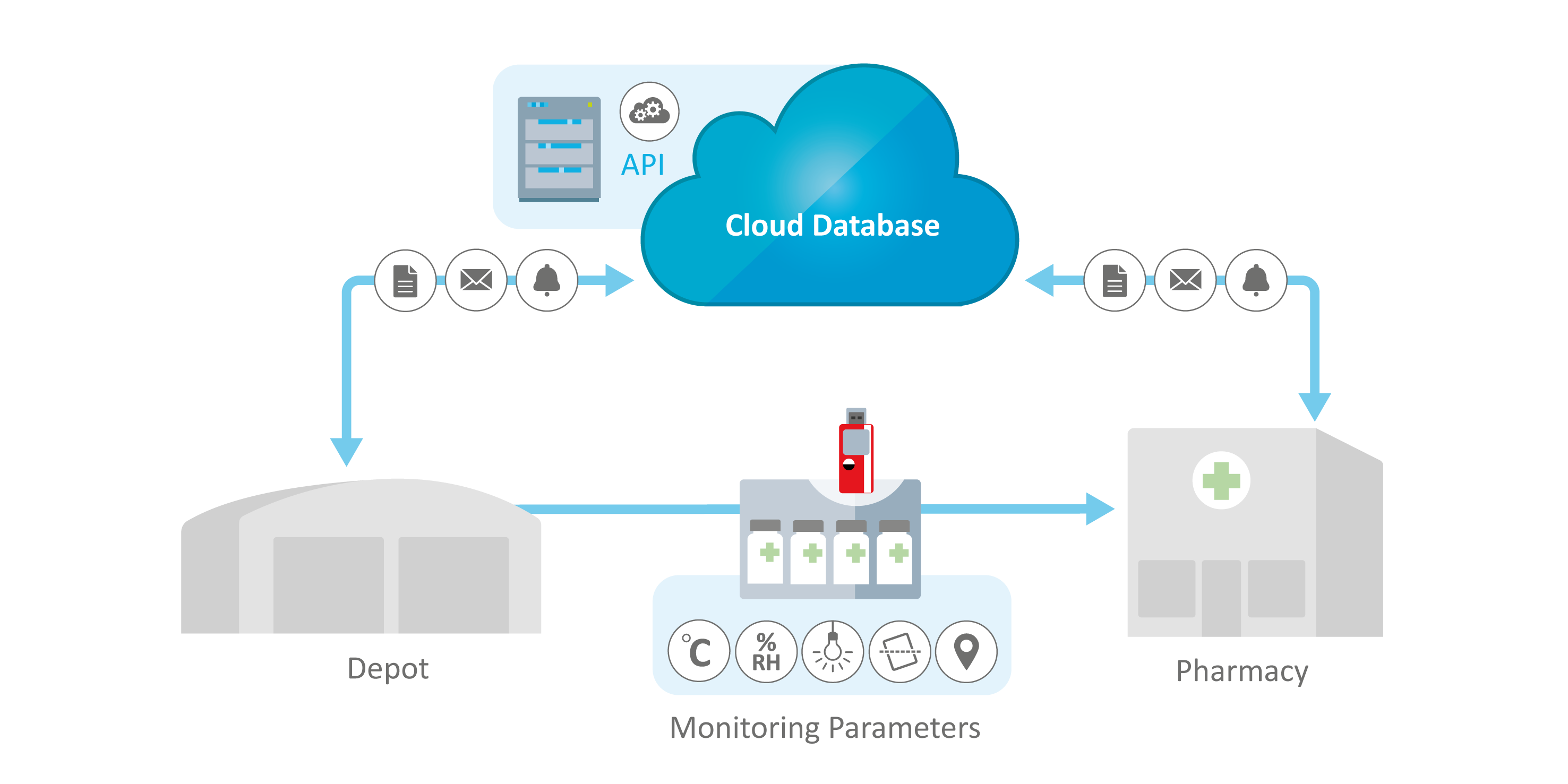
Direct-to-Patient Monitoring Applications
ELPRO supports Direct-to-Patient approaches with validated environmental monitoring solutions that document and verify end-to-end product integrity and compliance—providing the critical data needed to confirm product quality and support patient safety.
-
Site-to-Patient Deliveries
-
Depot-to-Patient
-
Hybrid Drug Delivery Models

Monitor Trial Shipments from Study Sites to Patients or Physicians
In the site-to-patient model, study drugs are stored at clinical sites and then delivered directly to participants or to a physician participating in the study. ELPRO’s single-use USB PDF data loggers and smart indicators verify that medications remain within safe temperature ranges, giving both site staff and patients confidence in product quality—all while meeting GxP and protocol requirements. Leading global CROs and CDMOs trust that site-to-patient deliveries with ELPRO solutions will always meet safety and efficacy standards.

Ensure Stability with Seamless Monitoring
Managing complex, multi-step deliveries from depot to patient pose data integration risk across diverse systems. ELPRO's monitoring solutions integrate data from various devices (Bluetooth®, USB data loggers, etc.) into one software solution. By this, relevant data is made available to your enterprise systems and dashboards, ensuring that all environmental conditions can be tracked and reconciled in a single, unified source.
In direct-to-patient deliveries it is crucial that the product's usability is clearly visible at kit level. ELPRO’s smart indicators show the current status visually and simply using the LED indicators: Green = OK, Red = ALARM. In this way, they help patients to use only compliant products.

Support Flexible Trial Designs with Site Visits and Home Delivery
Hybrid CRT drug delivery models combines Depot-to-Patient (DtP) delivery with traditional site visits for critical procedures or interventions, offering flexibility in clinical trials. ELPRO's solutions ensure that investigational drug products are consistently tracked throughout both delivery methods and provides a unified view of product stability. This ensures compliance, reduces the risk of undetected temperature excursions and ensure patient safety.
Simplify your Direct-to-Patient Logistics
Make faster, better-informed decisions throughout complex direct-to-patient trials shipments. Take advantage of real-time analytics and our data and process management software liberoMANAGER with more powerful tools:
- Stability budget management
- Single or multi-stage supply chain management
- Real-time alarms and notifications
- Automated reporting & assessment
- Advanced user / access management
- Customizable workflows
- Auto assessments and software-based alarming for mixed-load shipments
- API integration for seamless data transfer to other systems


Single-Use USB
- Temperature
- Automated PDF report generation
- USB connection

Cellular IoT Real-Time
- Temperature, humidity and position
- Real-time data generation and alarming
- Single-Use / Multi-Use

Smart Digital Indicator
- Visual alarm status
- Thin, small and self-adhesive
- LED Status for Go/No-Go decisions
We have new ideas collaborating with ELPRO for the future. They have supported our goals to improve our supply chain. Together with ELPRO, we are confident we will keep working out the best solutions.
Compliance Made Easier
Navigating global regulations and standards can be complex—but with expertise, it doesn’t have to be. Comprehensive solutions are provided to keep companies compliant and prepared for upcoming audits, ensuring smooth operations worldwide at all times. The complexity is handled, allowing focus on what matters most—your success.

Direct-to-Patient Logistics Monitoring? Contact Us Today.

Common Questions About Direct-to-Patient Logistics
When it comes to temperature-sensitive products, Direct-to-Patient (DtP) monitoring introduces new logistical and compliance challenges. Below, we’ve answered common questions about how ELPRO supports DtP strategies with reliable, regulatory-compliant environmental monitoring solutions that protect product quality and simplify trial execution.Why is environmental monitoring important for Direct-to-Patient (DtP) clinical trials?
Temperature-sensitive drugs and diagnostics can lose efficacy when exposed to out-of-range conditions during shipment. ELPRO’s validated data loggers and smart indicators ensure full traceability and documentation from the point of origin to the patient’s door—helping sponsors protect product integrity and remain compliant with GxP requirements.
What types of temperature monitoring devices does ELPRO offer for DtP logistics?
ELPRO offers a range of single-use USB PDF loggers, real-time loggers, and smart indicators designed for kit-level monitoring. These devices are easy to use for patients and caregivers, while providing robust data for sponsors to meet audit, reporting, and stability budget needs.
Can patients use ELPRO data loggers without special training?
Yes. ELPRO’s single-use USB loggers and indicators are designed for simplicity. Patients can easily read a clear “OK”/“Not OK” status or plug the logger into a USB port to access a PDF report—no software or training required.
What happens if there’s a temperature excursion during delivery?
ELPRO loggers automatically detect and record excursions, so sponsors can quickly assess whether the product remains usable. Our monitoring software helps streamline decision-making by correlating excursion data with the product’s known stability budget.
How does ELPRO help with cross-border depot-to-patient shipments?
For international deliveries, ELPRO’s real-time and PDF loggers ensure temperature conditions are maintained and documented, even across customs and varied transit times. This helps sponsors maintain regulatory compliance in multiple countries while minimizing delays and costly product losses.
What kind of data access and integration does ELPRO provide?
Monitoring data from ELPRO devices is accessible via secure cloud platforms or integrated into your clinical systems. Our LIBERO Cx, LIBERO Gx and liberoMANAGER software solutions support centralized data access, automated alarming and simplified reporting across global trials.
Can ELPRO support hybrid trial models that combine site visits with home delivery?
Absolutely. ELPRO offers flexible monitoring solutions that work across site-to-patient, depot-to-patient and hybrid models. Our devices track conditions across all phases of delivery and storage—ensuring consistent quality and documentation, whether drugs are stored onsite or shipped directly.
How does ELPRO simplify monitoring at the kit level?
ELPRO’s data loggers and indicators can be integrated at the kit level during packaging, enabling continuous monitoring of each shipment throughout the supply chain. This ensures visibility not just at a batch level, but down to individual patient kits—ideal for personalized therapies and small-batch drugs.
Why partner with ELPRO for your Direct-to-Patient clinical trials?
ELPRO combines decades of experience in GxP-compliant monitoring with innovative, easy-to-use technology tailored for decentralized trials. We offer global support, seamless integration with your trial workflows, and a commitment to helping you protect product quality and improve patient safety at every step.
How does ELPRO support compliance with regulatory standards?
ELPRO’s monitoring solutions are fully compliant with FDA 21 CFR Part 11, GAMP® 5, and global GxP guidelines. Our systems ensure secure data handling, audit trails and validation documentation to support regulatory inspections and streamline sponsor oversight.
What does the term 'last-mile approach' mean in logistics?
In logistics, the "last mile" refers to the final step of the delivery process—when a product is transported from a distribution center, depot, or local hub to its final destination, such as a customer’s home, a retail store, a hospital, or a clinical site.
In pharmaceutical or clinical trial logistics, the "last mile" often means the delivery from a local depot or clinical site to a patient or physician, and it is especially critical because it involves small volumes, strict temperature requirements, and the need for timely, secure, and documented delivery. It is called "last mile" even if the distance is more or less than one actual mile.
Newsletter-Sign Up ELPRO News
ELPRO will use the information provided in this form to keep in touch with you and to send you updates and marketing information by e-mail.


