GxP Compliance Consulting & Documentation Services
Remain Compliant with ELPRO's GxP Services
The complexity of regulatory requirements makes ensuring compliance tedious and time-consuming. GxP compliance consulting and qualification services take this burden off your shoulders—ensuring preparedness for all upcoming audits.
Pharma, biotech companies, medtech firms, and other life sciences organizations rely on expert GxP compliance services to reduce inspection risk, maintain data integrity, and accelerate compliant product development. GxP consulting and qualification services take this burden off your shoulders—ensuring preparedness for all upcoming audits.
Covering rooms and warehouses, equipment, systems and fleets, the services include:
- Equipment qualification
- Transport validation and verification
- Warehouse and room qualification
- System validation
-
GxP Consulting Services
-
GxP Qualification & Validation
-
GxP Mappings

GxP Consulting Services
Customers profit from four decades of expertise in pharmaceutical environment control and GxP compliance guidance. ELPRO consultants provide step-by-step support to navigate complex regulations, improve processes, scale for future growth, and develop strategies to ensure compliance, regardless of the size of current operations.
Whether you need to remediate your quality management system (QMS), prepare documentation for an upcoming FDA or EMA inspection, or establish scalable GxP compliance services, ELPRO's consultants deliver practical, audit-ready solutions.

GxP Qualification and Validation
Meticulous qualification services ensure that equipment and environmental control systems meet stringent GxP standards. Risk assessments, DQ, IQ, OQ, and PQ documentation are provided, guaranteeing the integrity of operations and protecting valuable assets.
The offer includes:
- Qualification of storage and transport units
- Validation/verification of transport processes
- Temperature and humidity mapping, including stress tests
- Creation of individual qualification/validation documentation

GxP Temperature and Humidity Mappings
Accurate environmental mapping is crucial in maintaining the conditions necessary for GxP compliance. ELPRO’s services provide in-depth mappings of environmental parameters, especially temperature and relative humidity, ensuring product quality and regulatory adherence. They include:
- Storage or cleanrooms
- Containers
- Thermal packaging or boxes
- Refrigerators, freezers or ULTs
- Trucks, vans or entire vehicle fleets
- Lane studies
Choose from onsite mapping services, remote mapping services or do-it-yourself mapping kits.
From temperature mapping to compliance in two months
Learn how one of Germany’s leading online pharmacies successfully completed a GMP-compliant temperature mapping in its highly automated AutoStore system. Get insights into the challenges, process, and results—and learn how precise monitoring supports regulatory compliance and product safety.
Always in Mind: the V-Model as Compliance Guide
The V-model enables compliance, and you can choose the phase where our expert knowledge best supports your GxP compliance needs.
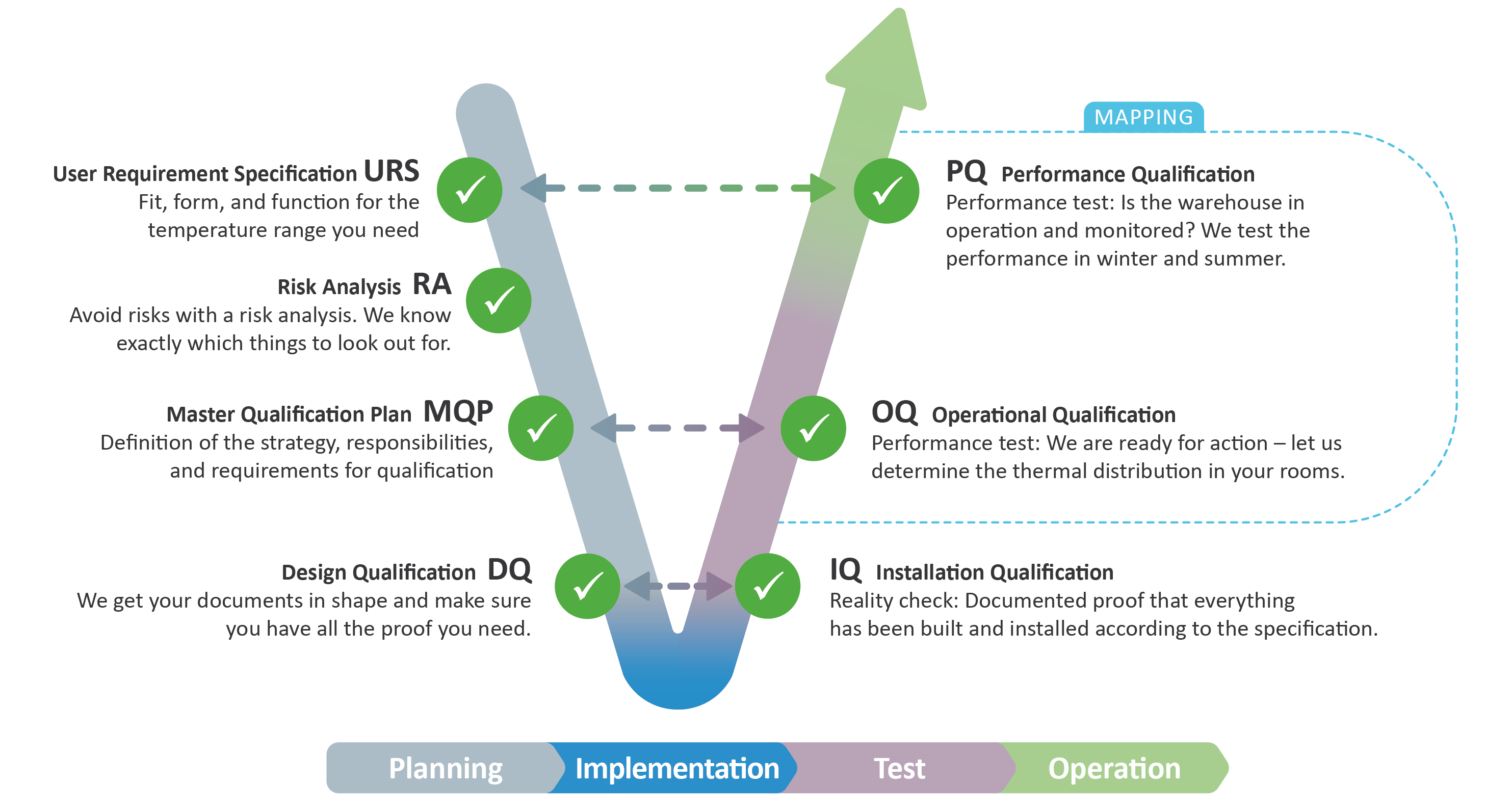
Risk-Based GxP Services and Qualification for Monitoring Systems
A risk-based approach is fundamental to effective GxP compliance consulting. ELPRO applies structured risk methodologies to define the appropriate scope, depth and documentation requirements for every qualification and validation project.
- Structured risk assessments to define qualification scope and validation depth, ensuring resources are focused where they matter most for regulatory compliance
- Identification of critical parameters and system components impacting compliance, from temperature sensors and data loggers to software interfaces and alarm systems
- Documentation aligned with regulatory expectations and inspection readiness, including risk assessment reports, qualification protocols (IQ/OQ/PQ), and summary reports that satisfy FDA, EMA and WHO reviewers
Lifecycle Approach to GxP Services and Qualification for Monitoring Systems
Compliance is not a one-time event. It requires a disciplined lifecycle approach. ELPRO's GxP compliance services are designed to support your monitoring systems from initial design through ongoing operation, ensuring continuous regulatory adherence.
- End-to-end qualification strategy from system design through operation and maintenance, aligned with the V-model framework and current regulatory expectations
- Periodic review and requalification to ensure ongoing compliance as regulations, facility conditions or operational requirements evolve
- Change control support to manage system updates within regulated environments, ensuring that hardware upgrades, software changes, or facility modifications are properly assessed and documented within your QMS
Keeping Your Environmental Monitoring System Compliant
Logistics, clinical trials and biopharma companies worldwide trust in ELPRO's GxP Service expert.
500+
Warehouses Qualified
10,000+
Facilities Temperature Mapped
20,000+
Calibrations Annually
ELPRO facilitated the qualification of both the temperature monitoring equipment as well as our cold storage units by providing excellent expertise and technical support just in time.
We had a really fruitful collaboration with ELPRO concerning the qualification and mapping of our new GMP/GDP warehouse. All steps until the authority inspection were attended professionally and finally, we had no issues.
GDP Compliance: Unmatched GxP Support throughout Your Entire Supply Chain
GxP compliance services must support the entire supply chain to ensure continuous temperature control and compliance:
- Tailored solutions maintain the critical temperature range for life-saving, temperature-sensitive products.
- Cloud-based solutions provide real-time monitoring and data insights for seamless tracking.
- Experts mitigate non-compliance risks, ensuring product safety and full regulatory adherence from storage to distribution.
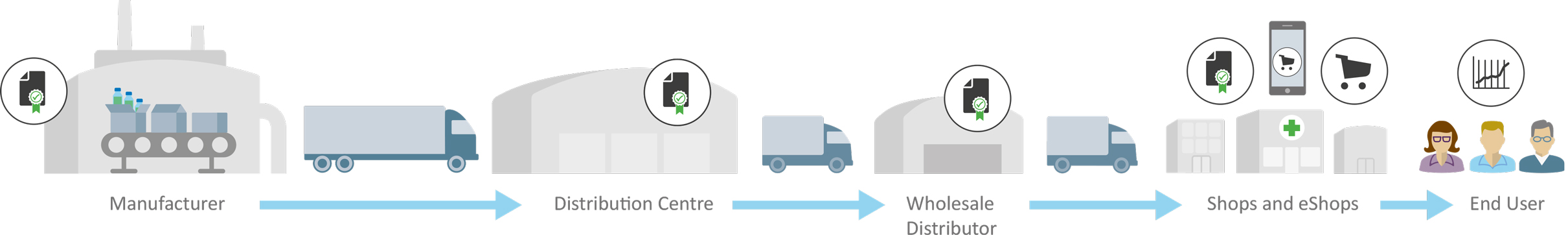
Compliance Made Easier
Navigating global regulations and standards can be complex—but with expertise, it doesn’t have to be. Comprehensive GxP compliance consulting and documentation services are provided to keep companies compliant and prepared for upcoming audits, ensuring smooth operations worldwide at all times. The complexity is handled, allowing focus on what matters most—your success.

Let's talk GxP. Contact us today.

Frequently Asked Questions About GxP Services, GxP Consulting and Qqualification
What does GxP stand for?
GxP stands for "Good x Practice," where x represents different fields such as Manufacturing (GMP), Laboratory (GLP), and Distribution (GDP). It is a general term for regulations and guidelines ensuring quality, safety, and compliance in industries like pharmaceuticals, biotechnology, and healthcare.
Some common GxP standards include:
- GMP (Good Manufacturing Practice) — Ensures products are consistently produced and controlled according to quality standards.
- GLP (Good Laboratory Practice) — Ensures the reliability and integrity of laboratory studies.
- GDP (Good Distribution Practice) — Ensures proper handling, storage, and transport of pharmaceutical products.
- GCP (Good Clinical Practice) — Ensures the ethical conduct, safety, and reliability of clinical trials while protecting the rights and well-being of participants.
These guidelines help maintain product quality, regulatory compliance and patient safety.
What is a GxP validated system?
A GxP-validated system is a computerized system that has been tested and documented to ensure it meets regulatory requirements for data integrity, accuracy, reliability, and security in GxP environments (such as GMP, GLP, or GDP). Key aspects of a GxP validated system are:
- Compliance with regulations — The system must meet regulatory requirements such as FDA 21 CFR Part 11, EU Annex 11, and other industry-specific guidelines.
- Validation process (CSV – Computerized System Validation) — The system undergoes Installation Qualification (IQ), Operational Qualification (OQ) and Performance Qualification (PQ) to ensure it works as intended.
- Data integrity (ALCOA+ Principles) — Ensures data is Attributable, Legible, Contemporaneous, Original, Accurate, and includes additional requirements like Complete, Consistent, Enduring and Available.
- Audit trail & security — The system must track all changes, ensure user access control, and prevent unauthorized modifications.
- Documented evidence — Validation must be fully documented to demonstrate compliance during audits and inspections. This documentation typically includes a Validation Plan, IQ/OQ/PQ protocols and execution reports, a Traceability Matrix, and a final Validation Summary Report.
What are GxP regulatory requirements?
GxP regulatory requirements are guidelines and standards ensuring product quality, safety, and data integrity in industries like pharmaceuticals, biotechnology, and healthcare. These regulations vary by region and application but share common principles. Key GxP regulations are:
1. Manufacturing & Laboratory Regulations
- EU GMP Guidelines (EudraLex Volume 4)
- US FDA 21 CFR Part 210 & 211
- WHO Guidelines
- OECD Principles of GLP
- US FDA 21 CFR Part 58
2. Distribution & Supply Chain Regulations
- EU GDP Guidelines (2013/C 343/01)
- US FDA GDP Guidance for Industry
- WHO Guidelines
-
ISPE Good Practice Guide
3. Data Integrity & Electronic Records Regulations
- FDA 21 CFR Part 11—Defines requirements for electronic records and signatures to ensure data integrity.
- EU Annex 11—Covers computerized systems in GxP environments.
- MHRA & WHO Data Integrity Guidelines—Emphasize compliance with ALCOA+ principles (Attributable, Legible, Contemporaneous, Original, Accurate, plus Complete, Consistent, Enduring, Available).
4. Medical Device & Clinical Trial Regulations
- ISO 13485—Quality management system for medical devices.
- ICH GCP (Good Clinical Practice) Guidelines—Ensures ethical and scientific standards in clinical trials.
What are typical GxP services?
Typical GxP services ensure compliance with regulatory standards in industries like pharmaceuticals, biotechnology, and healthcare. These services include:
1. Qualification & Validation Services
- User Requirements Specification
- Risk Assessment
- Temperature Mapping—Ensuring controlled environments (e.g., warehouses, refrigerators, freezers) meet GxP requirements.
- Equipment Qualification (IQ/OQ/PQ)—Installation, operational and performance qualification of monitoring and storage systems.
2. Monitoring & Compliance Solutions
- Environmental Monitoring (Temperature, Humidity, CO₂, etc.)—Continuous monitoring of storage and transport conditions.
- Deviation Management & Alarm Handling—Ensuring timely response to out-of-specification conditions.
- Audit Trail & Data Integrity Assurance—Secure data logging and documentation for compliance.
3. GxP Consulting & Audits
- Regulatory Compliance Support—Guidance on GMP, GDP and other GxP requirements. This includes gap analysis against current regulatory expectations, quality system remediation, and preparation of controlled documentation such as SOPs, qualification protocols and validation reports.
- Risk Assessments & Gap Analysis—Identifying potential compliance risks and improvement areas.
- Audit & Inspection Readiness—Preparing for regulatory inspections (e.g., FDA, EMA).
4. Training & Documentation
- GxP Training for Personnel—Educating staff on regulatory requirements and best practices.
- Standard Operating Procedures (SOPs) Development—Creating documented guidelines for compliance. Other GxP documentation services include the creation or review of batch records, qualification protocols, validation plans, change control procedures and deviation/CAPA documentation to support a robust QMS.
These services help companies maintain compliance, ensure product quality, and safeguard patient safety.
What are three GxP relevant elements?
The three basic elements of GxP are:
- Traceability—The ability to track the development, manufacturing, testing, and distribution of a product to ensure transparency and accountability.
- Accountability—Clearly defining responsibilities and documenting actions to ensure compliance with regulations and standards.
- Data Integrity—Ensuring that data is accurate, complete, consistent, and secure throughout its lifecycle to support regulatory compliance and decision-making.
These principles help maintain product quality, regulatory adherence, and patient safety in industries like pharmaceuticals and healthcare.
What role does the V-model play in qualification?
The V-Model is a structured approach used in GxP qualification and validation to ensure that equipment, facilities, and computerized systems meet regulatory requirements. It is widely applied in Good Manufacturing Practice (GMP), Good Laboratory Practice (GLP), and Good Distribution Practice (GDP) environments.
The V-Model follows a "V" shape, where:
- The left side defines requirements and design.
- The right side verifies and validates the system against those requirements.
- The middle (implementation phase) represents the system’s installation and configuration.
Each step on the left has a corresponding testing phase on the right, ensuring full traceability and compliance.
What are the 5 R's of risk assessment?
The 5 R’s of risk assessment provide a structured approach to identifying and managing risks effectively. They help organizations, especially in regulated industries like pharmaceuticals, maintain compliance and minimize potential failures. Using the 5 R’s ensures a proactive and systematic approach to managing risk in highly regulated environments.
- Recognize—Identify potential risks that could impact product quality, patient safety, or regulatory compliance. This involves analyzing processes, equipment, and data integrity vulnerabilities.
- Rank—Assess and prioritize risks based on their likelihood, severity, and detectability. Methods like FMEA (Failure Modes and Effects Analysis) help determine which risks require immediate action.
- Respond—Develop and implement risk control measures, such as process improvements, training, system validations, or additional monitoring, to eliminate or mitigate risks.
- Record—Document risk assessments, mitigation strategies, and corrective actions to ensure GxP compliance with industry regulations like CFR 21 Part 11, ICH Q9, and ISO 14971.
- Review—Continuously monitor risks and update assessments to account for changes in processes, regulations, or new threats. Periodic reviews ensure long-term compliance and operational safety.
What is the difference between CSV and GxP?
GxP is a broad term encompassing all "Good Practice" regulations—GMP, GLP, GDP, GCP and others—that govern how life sciences organizations develop, manufacture, distribute and document their products and processes. CSV, or Computerized System Validation, is a specific activity performed within the GxP framework to demonstrate that a computerized system consistently does what it is intended to do.
In practical terms, GxP defines the regulatory expectations; CSV is the documented process of meeting those expectations for software and IT systems. CSV follows a structured lifecycle—including User Requirements Specifications (URS), risk assessment, and IQ/OQ/PQ testing—and produces the documented evidence needed to satisfy regulators that a system is fit for its intended GxP use. For biotech companies and other life sciences organizations, integrating CSV into your QMS is essential to ensuring both system reliability and ongoing regulatory compliance.
What documentation is required for GxP services and qualification of environmental monitoring systems?
Proper GxP documentation services are critical to demonstrating compliance during regulatory inspections. For the qualification of environmental monitoring systems, the following documents are typically required:
- Validation Master Plan (VMP) or Qualification Plan — Defines the overall strategy, scope and responsibilities for the qualification project.
- User Requirements Specification (URS) — Describes what the system must do from a user and regulatory perspective.
- Risk Assessment — Identifies and prioritizes critical system components and parameters.
- Installation Qualification (IQ) Protocol & Report — Verifies the system is installed correctly per manufacturer specifications and site requirements.
- Operational Qualification (OQ) Protocol & Report — Confirms the system operates as intended across its defined operating range.
- Performance Qualification (PQ) Protocol & Report — Demonstrates consistent system performance under real-world operating conditions.
- Temperature Mapping Report — Documents the spatial temperature distribution within a monitored environment.
- Calibration Certificates — Evidence that sensors and instruments meet required measurement accuracy standards.
- Traceability Matrix — Links each user requirement to a corresponding test, ensuring full coverage.
- Validation Summary Report — Consolidates all qualification activities and formally concludes the validation lifecycle.
- Standard Operating Procedures (SOPs) — Documented procedures for system operation, alarm response, deviation handling and periodic review.
How long does a typical GxP qualification process for environmental monitoring systems take?
The timeline for GxP qualification of an environmental monitoring system varies depending on the scope, complexity, and current state of documentation. As a general guide:
- Small or single-room qualifications (e.g., a single cold room or laboratory): 2-6 weeks, including documentation, mapping and reporting.
- Mid-scale projects (e.g., a multi-zone GDP warehouse or clinical storage facility): 6-12 weeks, factoring in risk assessment, full IQ/OQ/PQ execution, and regulatory review cycles.
- Large or complex environments (e.g., a highly automated distribution center or multi-site rollout): 3-6 months or more, particularly when QMS integration, change control and authority inspection preparation are included.
Timelines can be compressed when existing documentation is well-organized and a clear qualification strategy is in place. ELPRO's GxP compliance consulting team works with your quality and operations teams from the outset to scope projects accurately, allocate resources efficiently, and meet your audit or go-live deadlines.
Newsletter-Sign Up ELPRO News
ELPRO will use the information provided in this form to keep in touch with you and to send you updates and marketing information by e-mail.


