Pharmacy & Hospital
Secure Temperature Monitoring Systems for Pharmacies & Hospitals
Ensure compliance and continuous oversight with ELPRO’s environmental monitoring systems designed for pharmacies, hospitals, and clinics. Whether independent or as part of an integrated enterprise solution, the system adapts easily to different facility types and standard operating procedures. Real-time data from rooms and equipment is accessible anytime, anywhere—making it easier to stay in control and respond quickly to deviations.
- Real-time monitoring with wireless sensors and cloud-based access
- Immediate alerts via email and/or SMS
- Easy to set up, modify, and expand as needs grow
- Fully compliant with FDA 21 CFR Part 11 and GAMP® 5 standards
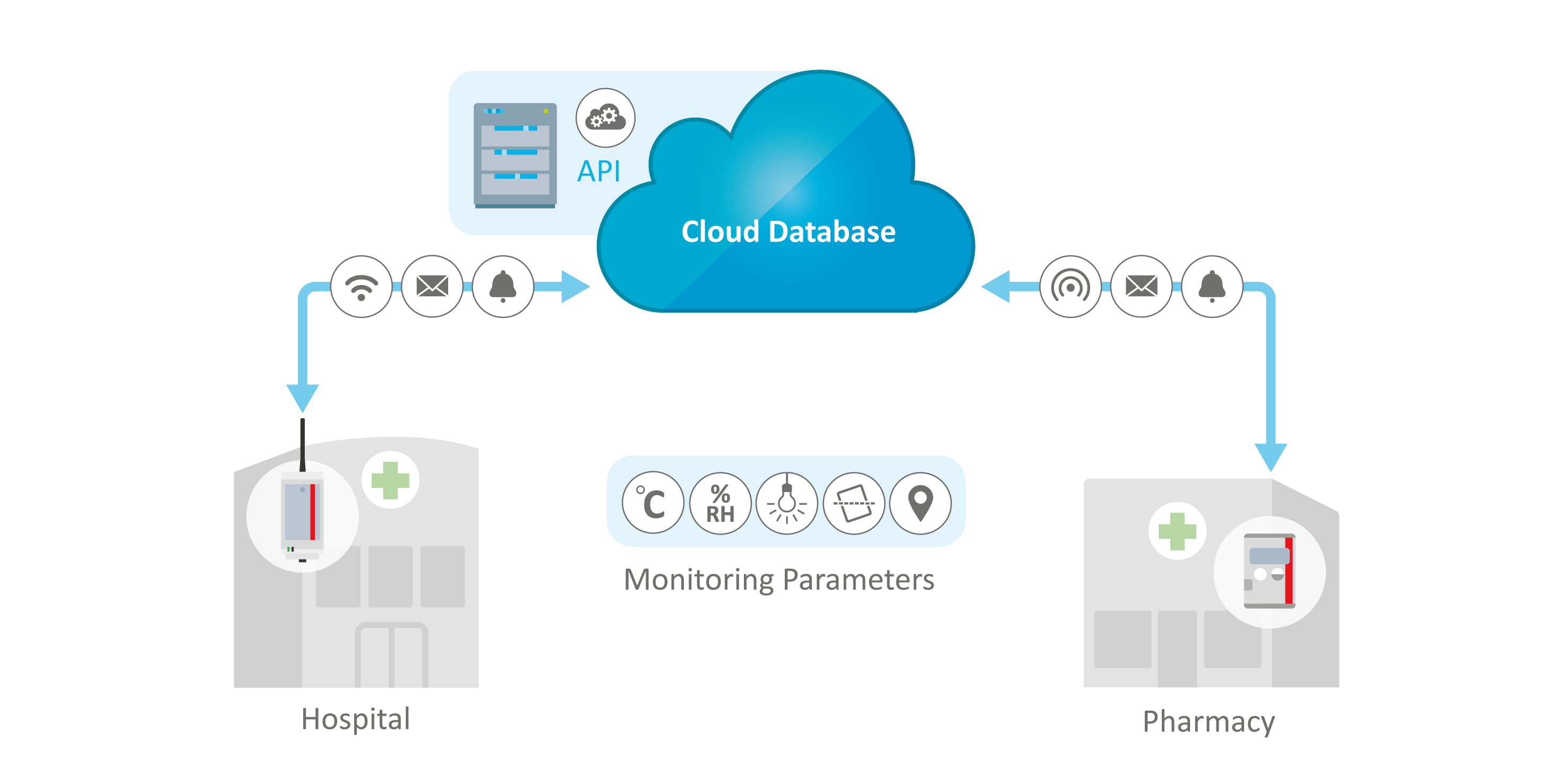
Protect Medications and Therapies at Every Site
Every type of distribution site has unique requirements. For trusted pharmacy temperature monitoring, ELPRO wired and wireless humidity and temperature monitoring systems for pharmacies are scalable can be customized to meet your requirements and SOPs.
-
Retail & Specialty Pharmacies
-
Central Hospital & Clinic Pharmacies
-
Clinical Research Pharmacies

Retail & Specialty Pharmacies
Retail pharmacies store a range of medications, including vaccines, biologics, and oral treatments that require specific temperature conditions. Environmental monitoring helps protect temperature-sensitive products such as vaccines, insulin, biologics, and other injectable medications.

Central Hospital & Clinic Pharmacies
Central pharmacies in hospital systems and even in smaller clinics often store a wide range of medications, including critical care medications, some of which are temperature-sensitive. Monitoring systems are fundamental to protect temperature-sensitive products such as intravenous (IV) medications, chemotherapy drugs, blood products, and vaccines.

Clinical Research Pharmacies
Clinical research pharmacies play a crucial role in storing and managing medications for clinical trials, many of which are temperature-sensitive and require precise conditions. ELPRO solutions ensure the integrity of these vital products, including investigational drugs, biologics, and vaccines, by providing reliable temperature monitoring to safeguard their efficacy and compliance throughout the research process.
Cloud-Based Real-Time Monitoring Made Easy
- Radio modules and IoT data loggers to monitor rooms and equipment
- Global 24/7 access
- Easy to set up, modify and expand
- Immediate alerts via e-mail and/or SMS
- FDA 21 CFR Part 11 compliant and GAMP® 5 validated
- Unlimited number of sensors
- API for integration into your system (optional)


Cellular IoT Real-Time
- °C, %RH
- LTE-M and NB-IoT (global roaming)
- IT-independent installation
- Easily scalable

Wireless Modules
- °C, %RH, 4to20mA
- Radio frequency: 868 / 915 MHz
- Flexible positioning of sensors
- Up to 50 sensors per local radio bridge
Cloud Solution for Real-Time Temperature Monitoring
Learn how a hospital pharmacy implemented a GxP-compliant, IT-independent monitoring system with remote access that enabled cloud-based alarming, temperature monitoring without building system integration, and easy device scaling.
My fundamental trust in ELPRO’s solutions, which are GAMP® 5 validated and compliant with FDA 21 CFR Part 11, meant that the issues of data integrity and cloud-based solutions were never a matter of uncertainty for me. I had and have great confidence in ELPRO and its products.

Ensuring Safe Transport of Life-Saving Therapies
ELPRO monitoring solutions support both stationary storage and cold chain transport of temperature-sensitive products, such as personalized medicines and therapies. Trusted by leading white-glove couriers, these solutions ensure reliability across every stage—from facility to patient—when time, temperature, and security matter most.
- Monitor refrigerated transport across facilities with real-time visibility
- Protect product efficacy and prevent loss during transit
- Enable immediate intervention through live alerts
- Ensure full compliance and maintain data integrity throughout the journey
Compliance Made Easier
Navigating global regulations and standards can be complex—but with expertise, it doesn’t have to be. Comprehensive solutions are provided to keep companies compliant and prepared for upcoming audits, ensuring smooth operations worldwide at all times. The complexity is handled, allowing focus on what matters most—your success.

Let's Talk Healthcare Temperature Monitoring.

Common Questions About Monitoring Pharmacies & Hospitals
To select the right pharmacy temperature monitoring system for your facility, begin by asking the following questions:How is temperature and humidity maintained in a pharmacy?
Maintaining the correct temperature in pharmacies, including hospital central pharmacies, is crucial for drug stability and efficacy. Key practices include:
- Identifying Temperature Requirements: Medications must be stored at specific temperatures, such as refrigerated (2 °C to 8 °C), frozen (-20 °C or lower), or room temperature (15 °C to 25 °C), depending on the drug.
- Temperature-Controlled Equipment: Pharmacies use pharmaceutical-grade refrigerators, freezers, and HVAC systems to maintain stable environments. These systems are equipped with monitoring and alarm features.
- Real-Time Monitoring: Continuous temperature monitoring systems, including mobile IoT data loggers like the ELPRO LIBERO Gx, track conditions and send alerts if temperatures deviate. These systems ensure immediate action is taken to protect medications.
- Backup Systems: Backup power sources, such as generators and uninterruptible power supplies (UPS), ensure uninterrupted temperature control during power failures.
- Regular Maintenance: Equipment should be routinely checked and calibrated to ensure proper function, and storage units should be organized to prevent temperature fluctuations.
- Staff Training & SOPs: Pharmacy staff must be trained on temperature monitoring and emergency protocols. Standard Operating Procedures (SOPs) should guide responses to temperature excursions.
Does the FDA require temperature monitoring?
The FDA does require temperature monitoring for certain drugs, particularly those that are temperature-sensitive. The FDA mandates that pharmaceutical products, especially biologics, vaccines, and other temperature-sensitive medications, be stored and transported within specified temperature ranges to maintain their stability and efficacy.
The FDA's Guidance for Industry on "Good Manufacturing Practice (GMP) for Active Pharmaceutical Ingredients" and related guidelines on storage and transportation emphasize the need for proper environmental controls, including temperature monitoring solutions like elproMONITOR or liberoMANAGER, to ensure that medications are stored under appropriate conditions.
Additionally, compliance with regulatory standards from agencies like the FDA and EMA (European Medicines Agency) ensures the safety and integrity of pharmaceuticals. Both agencies outline requirements for temperature-controlled storage and the monitoring of storage conditions, particularly for biologics, vaccines, and investigational products, to prevent degradation and ensure product quality.
While the FDA's guidelines may not explicitly use the term "temperature monitoring" in every context, they clearly require that drugs, especially temperature-sensitive ones, are stored and transported under controlled conditions to maintain their quality, and that temperature deviations are tracked and managed appropriately.
What are the security measures necessary in a pharmacy for drug stability and efficacy?
In a pharmacy, especially where temperature-sensitive medications are stored or transported, security measures for temperature data monitoring are critical to maintaining drug stability and efficacy. These measures typically include:
- Real-Time Monitoring Systems: Pharmacies should implement continuous, real-time temperature and humidity monitoring systems with automated alerts. This ensures that any deviations from acceptable ranges are immediately detected, allowing for prompt corrective action to prevent damage to medications.
- Data Logging and Auditing: Secure data loggers should be used to capture detailed temperature data over time. This data should be stored in a secure, tamper-proof system that can generate reports for auditing purposes and ensure compliance with regulatory standards. It’s important that these logs are both accessible and protected from unauthorized access to maintain the integrity of the data.
- Access Control and Restriction: Only authorized personnel should have access to critical temperature monitoring systems and data. This can be achieved through secure login protocols, role-based access controls, and physical security measures like locked storage rooms and restricted areas.
- Backup Systems: Backup power systems (such as generators or uninterruptible power supplies) should be in place to prevent interruptions to temperature monitoring in the event of power failure. Additionally, cloud-based backups can ensure data is not lost during system malfunctions or local disruptions.
- Alarm and Notification Systems: Automated alarms should be configured to notify pharmacy staff or security personnel of temperature deviations or equipment malfunctions. These alarms should be sent via multiple channels (e.g., email, text, or phone calls) to ensure swift action can be taken.
- Environmental Control and Validation: Periodic validation of temperature-controlled areas, such as refrigerators or freezers, should be conducted to ensure that equipment is functioning correctly and that the storage conditions are consistent with manufacturer specifications. These validations help confirm that the drugs remain within the required stability parameters.
- Training and Protocols: Pharmacy staff should be trained on the importance of temperature monitoring, the proper response to temperature excursions, and the maintenance of equipment. Standard operating procedures (SOPs) should be in place to guide actions during any temperature-related incidents, ensuring proper handling and documentation.
Newsletter-Sign Up ELPRO News
ELPRO will use the information provided in this form to keep in touch with you and to send you updates and marketing information by e-mail.


