Smarter Supply Chains with Real-Time Visibility and Leaner Packaging
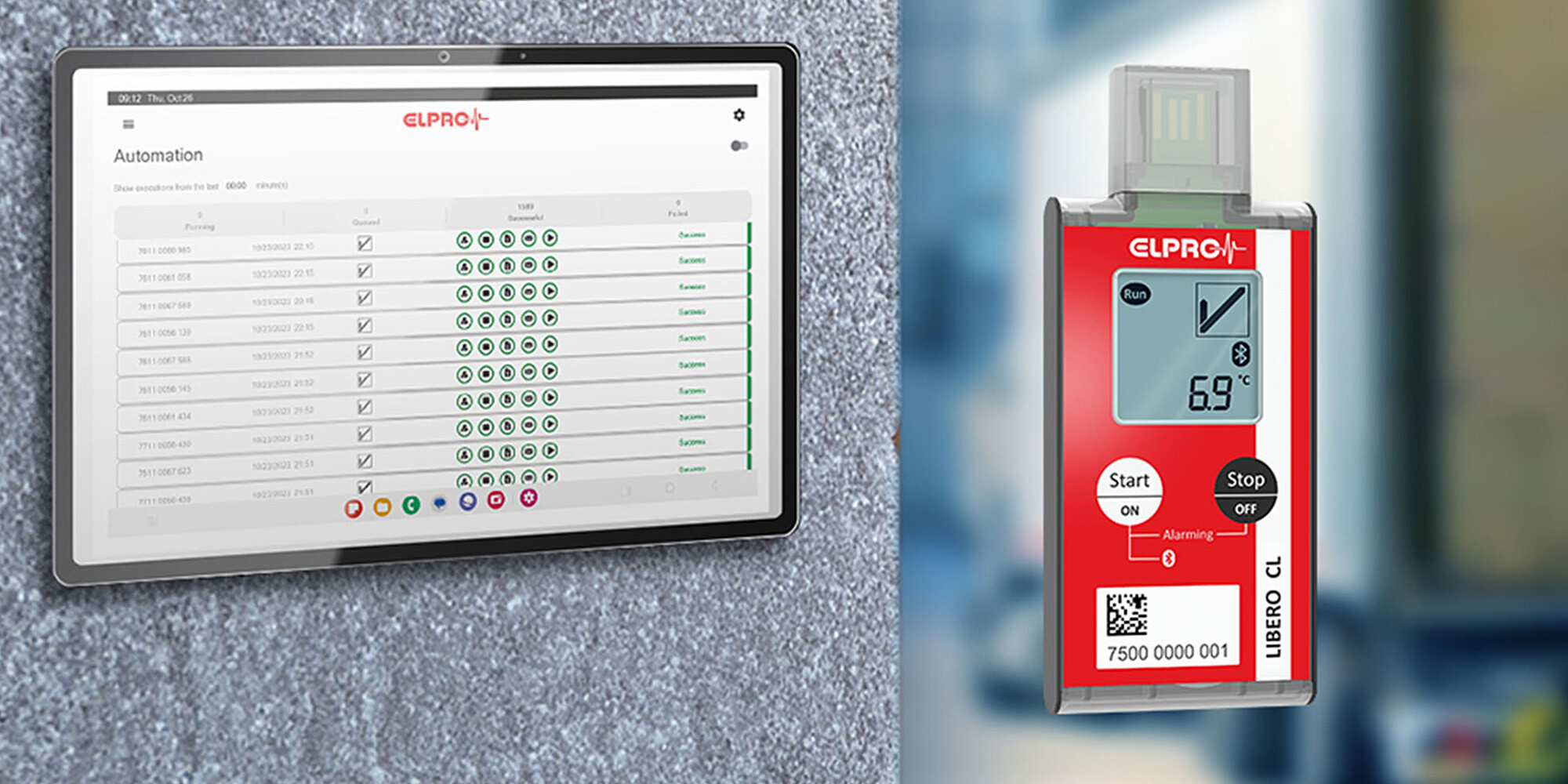
Real-time data loggers bring new opportunities for optimizing pharmaceutical supply chains. By continuously collecting data on product status and geo-location, they enable detailed analysis of where issues occur during transport. This information supports improvements in route planning, selection of logistics providers, and packaging design.
In addition, the availability of real-time alerts reduces dependency on costly active or passive packaging. More shipments can be made using cartonboard packaging, with real-time monitoring serving as a safety net. This shift contributes to cost efficiency and has a positive environmental impact by reducing packaging waste and preventing unnecessary product disposal.