Dry Ice Monitoring
Temperature Monitoring with Dry Ice Data Loggers
When shipping critical pharmaceuticals, temperatures often must be maintained below -60 °C or even -150 °C. Dry ice is commonly used in these extreme cold chain requirements. Specialized dry ice temperature monitoring data loggers are specifically designed to monitor ultra-low temperature environments.
- Precise dry ice temperature monitoring at extreme temperatures down to -80 °C
- Real-time alerts prevent costly product loss
- Specialized sensors designed for ultra-cold environments
- Audit-ready documentation simplifies regulatory compliance
- Seamless software integration enables complete cold chain visibility
Dry Ice Temperature Monitoring Applications
Temperature monitoring is critical in industries where maintaining ultra-low temperatures ensures product quality, patient safety and regulatory compliance. ELPRO’s dry ice temperature data loggers protect valuable materials every step of the way.
-
API & Vaccines
-
Biobanking
-
Clinical Trials
-
Freeze-Dried Products

Secure Critical Pharma Shipments Below -60 °C
Active pharmaceutical ingredients (APIs) and some vaccines require ultra-cold storage during transportation. Dry ice shipping with ELPRO data loggers ensures temperature compliance, safeguarding product quality throughout the cold chain and supporting safe, effective delivery to healthcare providers and patients worldwide.

Protect Irreplaceable Biological Samples in Transit
Tissue samples, stem cells and other biological materials stored in biobanks must be transported at sub-zero temperatures to retain viability. ELPRO dry ice temperature loggers offer dependable monitoring during transport, ensuring irreplaceable specimens are preserved from collection site to research lab or storage facility.

Preserve Accuracy in Clinical Sample Logistics
Clinical trial samples, including blood, plasma and biopsies, must be maintained at ultra-low temperatures to ensure integrity and accurate analysis. Dry ice temperature data loggers track temperature conditions in real time, giving CROs and sponsors confidence in every shipment and supporting compliance with GxP guidelines.

Maintain Stability for Lyophilized Drug Formulations
Freeze-dried drugs can degrade if exposed to temperature deviations during transit. ELPRO’s dry ice-compatible loggers monitor shipments in real time, ensuring lyophilized pharmaceuticals remain stable and effective from manufacturer to market—even under the harshest shipping conditions.
Benefits of Using Temperature Data Loggers for Dry Ice Shipments
Complete, Compliant Solutions for Ultra-Cold Dry Ice Shipments
ELPRO’s dry ice monitoring solution combines high-performance data loggers with powerful, intuitive software to protect ultra-sensitive pharmaceutical products during transit. From APIs and cell therapies to clinical samples and freeze-dried drugs, our end-to-end system ensures full visibility, traceability and compliance—even at temperatures down to -200 °C.
- Designed for dry ice and cryogenic environments
- Internal and external probe options for flexible use
- USB, Bluetooth® and real-time connectivity
- Easy data access via elproCLOUD and liberoMANAGER
- Automated reports and PDF documentation
- Configurable alerts and tracking
- GAMP® 5 validated and 21 CFR Part 11 compliant
- IQ/OQ templates and free calibration certificates

Single-Use
- Direct dry ice placement
- Measurement range down to -95 °C (internal sensor)
- Automatic PDF report
- Detailed data analysis

Multi-Use
- Measurement range -200 °C to +400 °C
- Strong M8 connector
- USB or Bluetooth® connection
- Alkaline battery—IATA-compliant

Frozen
- Temperature and Position
- Probe: -200 °C to +400 °C; Internal sensor: -35°C to +55 °C
- Cellular IoT real-time
- Lithium battery

Dry-Ice
- Temperature and Position
- Probe: -200 °C to +400 °C; Internal sensor: 0 °C to +55 °C
- Cellular IoT real-time
- Alkaline battery—IATA-compliant
In projects like this, where time is of the essence and we rely heavily on the expertise of the supplier, soft factors play a major role in addition to meeting user requirements. ELPRO already convinced us from the very first discussions with their ready willingness to find solutions, their competence, reliability and openness.
Compliance Made Easier
Navigating global regulations and standards can be complex—but with expertise, it doesn’t have to be. Comprehensive solutions are provided to keep companies compliant and prepared for upcoming audits, ensuring smooth operations worldwide at all times. The complexity is handled, allowing focus on what matters most—your success.

Let's Talk Cold Chain Distribution. Contact Us Today.

Common Questions About Dry Ice Temperature Monitoring
Whether you’re shipping cell therapies, clinical trial samples, or ultra-cold vaccines, reliable dry ice temperature monitoring is essential to maintain product integrity and meet strict regulatory requirements. Here are common questions to consider when selecting a monitoring solution for dry ice shipments.How can I measure the temperature of dry ice and how often should it be checked?
Measuring the temperature:
- Dry ice is extremely cold (–78.5 °C / –109.3 °F), so never touch it directly with bare hands or standard thermometers.
- Use infrared (IR) thermometers or thermocouples designed for extreme cold.
Factors affecting dry ice temperature:
- Air exposure: Warm air entering a container accelerates sublimation.
- Poor insulation: Packaging that is not well insulated can cause faster temperature loss.
- Container opening: Frequently opening the container lets cold CO₂ gas escape and warm air in.
- Ambient temperature: High surrounding temperatures will increase the rate of sublimation.
Checking frequency:
- For short-term shipments, check temperature at the start and end of transport.
- For longer-term storage or sensitive products, use continuous temperature monitoring with data loggers to ensure stability.
What temperatures can dry ice data loggers monitor?
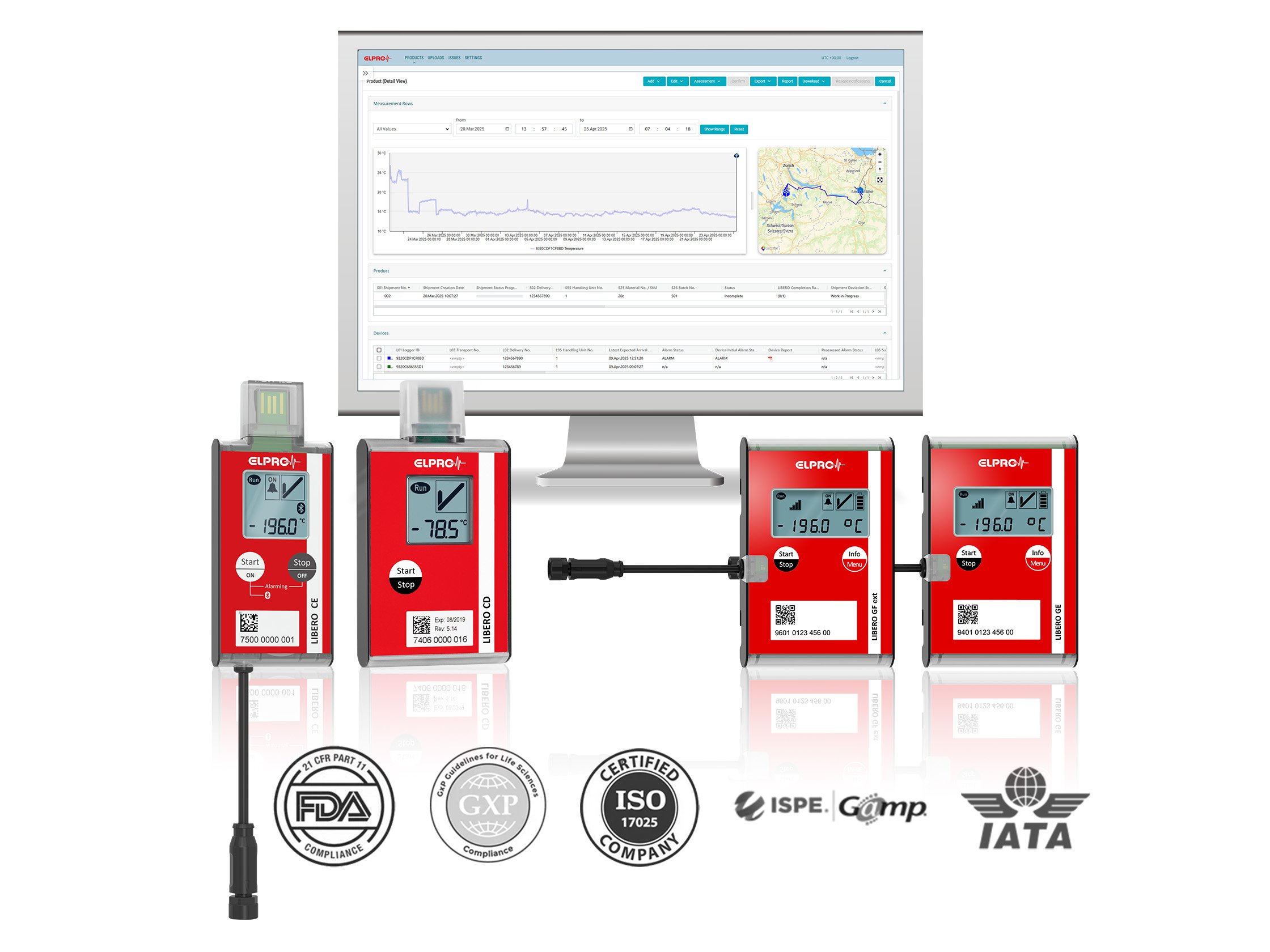
Dry ice data loggers typically monitor temperatures as low as -90 °C. ELPRO’s loggers—such as the LIBERO CE—are designed to reliably measure ultra-low temperatures, ensuring product safety during dry ice shipments.
Why is dry ice used in pharmaceutical shipping?
Dry ice maintains a stable temperature of -78.5 °C, ideal for transporting temperature-sensitive materials like cell therapies, APIs, and certain vaccines that must remain below -60 °C. ELPRO’s monitoring solutions help ensure these shipments stay within required temperature ranges.
What are the rules for transporting dry ice by air?
Dry ice is classified as a Dangerous Good (UN1845) under IATA regulations. The IATA guidelines state that shipments must comply with specific requirements:
- The package must be properly ventilated to allow gas release.
- The net weight of dry ice must be clearly marked on the package (e.g., “Dry Ice, UN1845, x kg”).
- Labels: Packages must display the Class 9 Miscellaneous Dangerous Goods label and the UN number.
- Documentation: Most shipments require a Shipper’s Declaration for Dangerous Goods, unless they qualify for specific exemptions.
- Training: Anyone preparing or offering dry ice for air transport must have IATA Dangerous Goods training.
- The limits for dry ice on an aircraft depend on the airline, type of aircraft, and shipment category (passenger vs. cargo aircraft).
Always check the current IATA Dangerous Goods Regulations (DGR) and the airline’s specific requirements before shipping.
Which ELPRO data loggers are suitable for dry ice shipments?
ELPRO recommends LIBERO CE, LIBERO CD, LIBERO GE, or LIBERO GF ext data loggers for dry ice applications. These models are validated for ultra-low temperatures and meet GxP requirements for dry ice monitoring logistics.
Are ELPRO dry ice data loggers GxP-compliant?
Yes. ELPRO’s dry ice data loggers are fully GxP-compliant and support 21 CFR Part 11 requirements. All data is tamper-proof and audit-ready, with automatic PDF reports and digital signatures for traceability.
Can ELPRO data loggers be reused?
Yes. ELPRO offers both single-use and multi-use options depending on your operational needs and shipment volume. Multi-use loggers are cost-effective for frequent shipments and long-term monitoring.
How is data accessed from a dry ice data logger?
Data from ELPRO data loggers can be transferred via USB, Bluetooth, or mobile networks to our cloud-based platform for real-time data access and storage – liberoMANAGER.
Can I get alerts during shipment?
Yes. For real-time monitoring, ELPRO’s LIBERO Gx data loggers can be paired with dry ice shipments to send instant alerts via e-mail or SMS if temperature thresholds are breached.
Are the data loggers easy to use for packaging teams?
Absolutely. ELPRO data loggers are pre-programmed and user-friendly, with simple activation buttons and clear LED status indicators. No technical training is needed, reducing error and saving time during shipment prep.
What happens if the temperature goes out of range?
ELPRO data loggers record every deviation and timestamp it. Alarms and LED indicators notify users immediately, and all excursions are clearly shown in the automatically generated PDF report for fast response and documentation.
Can ELPRO data loggers be used for international shipments?
Yes. ELPRO’s dry ice data loggers are approved for use on major airlines and comply with international shipping standards, making them ideal for cross-border logistics and global clinical trial distribution.
How do ELPRO solutions support regulatory audits?
ELPRO’s monitoring systems provide automated reporting, secure cloud backups, and validated software. This ensures audit readiness at all times, with full traceability and documentation that aligns with FDA, EMA, and WHO expectations.
What are the key safety measures when handling dry ice?
Dry ice, the solid form of carbon dioxide (CO₂), is commonly used to keep temperature-sensitive goods cold during transport. However, it must be handled with care.
Because dry ice is extremely cold (–78.5 °C), direct contact can cause frostbite — always use insulated gloves or tongs. As it sublimates, dry ice turns into CO₂ gas, which can displace oxygen in enclosed spaces, creating a risk of asphyxiation. Always work in well-ventilated areas and never store or transport dry ice in airtight containers, as gas buildup can cause them to rupture.
During air transport, dry ice is classified as a Dangerous Good (UN 1845) under IATA regulations. Shipments must be properly labelled and ventilated, and the net weight of dry ice clearly indicated on the package. Only trained personnel may prepare or offer dry ice shipments for air transport.
For more information, refer to official guidelines from OSHA (Occupational Safety and Health Administration) and IATA (International Air Transport Association).
Newsletter-Sign Up ELPRO News
ELPRO will use the information provided in this form to keep in touch with you and to send you updates and marketing information by e-mail.


