In clinical trials, each kit has its own history on its way from production to the clinical site—and behind every shipment are strict temperature requirements and tight time windows. In complex, multi-stage supply chains, even small deviations can jeopardize a kit's stability budget.
At the same time, conditions are becoming more challenging: smaller shipment sizes, decentralized and hybrid study designs, temperature-sensitive products, and increasing regulatory requirements are putting more pressure on processes along the entire cold chain.
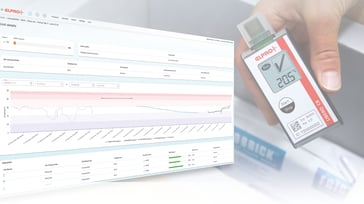
Until now, the remaining stability budget of a study kit was often calculated and evaluated manually—based on individual data loggers and fragmented transport information. This was time-consuming, error-prone, and difficult to scale. The future version of liberoMANAGER software closes this gap.
The future version of the software will, for the first time, enable fully kit‑based monitoring along the entire journey of a kit – from the production warehouse to the clinical site. Each kit will receive a unique identifier, allowing all temperature data from every transport segment to be automatically consolidated and evaluated. The remaining stability budget will always be transparent and easily accessible.
“We implement kit-based monitoring in liberoMANAGER in collaboration with a leading pharmaceutical company. Each transport route is evaluated individually and combined into an overall assessment based on the stability budget. This results in significantly fewer apparent deviations caused by strict temperature limits, allowing quality assurance to focus more efficiently on deviations that are truly relevant.”
Daniel Reichen, Global Key Account Manager, ELPRO
External temperature events – for example, from intermediate storage facilities or room monitoring systems – can also be added manually. liberoMANAGER immediately incorporates these inputs into the evaluation, ensuring that every decision is based on a complete and consistent data foundation.
Thanks to automated, rule-based assessments, kits that remain within the defined stability budget are released automatically, while any threshold exceedance triggers an automatic quarantine status. Through system interfaces, all results are transmitted directly to the IRT/RTSM environment, significantly accelerating study logistics and reducing manual steps.
The close interaction between ELPRO hardware and centralized software evaluation ensures that only truly critical kits are blocked. Unnecessary quarantines are avoided, study teams gain clarity faster, and the entire cold chain becomes more transparent and efficient.
Conclusion: Transparency and Traceability Throughout the Entire Study
With this approach, liberoMANAGER enables a continuous, traceable, and fully automated stability assessment for every kit across all transport stages – delivering greater efficiency, higher safety, and reliable compliance with regulatory requirements.
“With automated kit-level assessment and end-to-end data integration in liberoMANAGER, clinical teams can make decisions faster, minimize risks in a targeted manner, and ensure regulatory traceability at the same time.”
Simon Klein, Product Manager, ELPRO