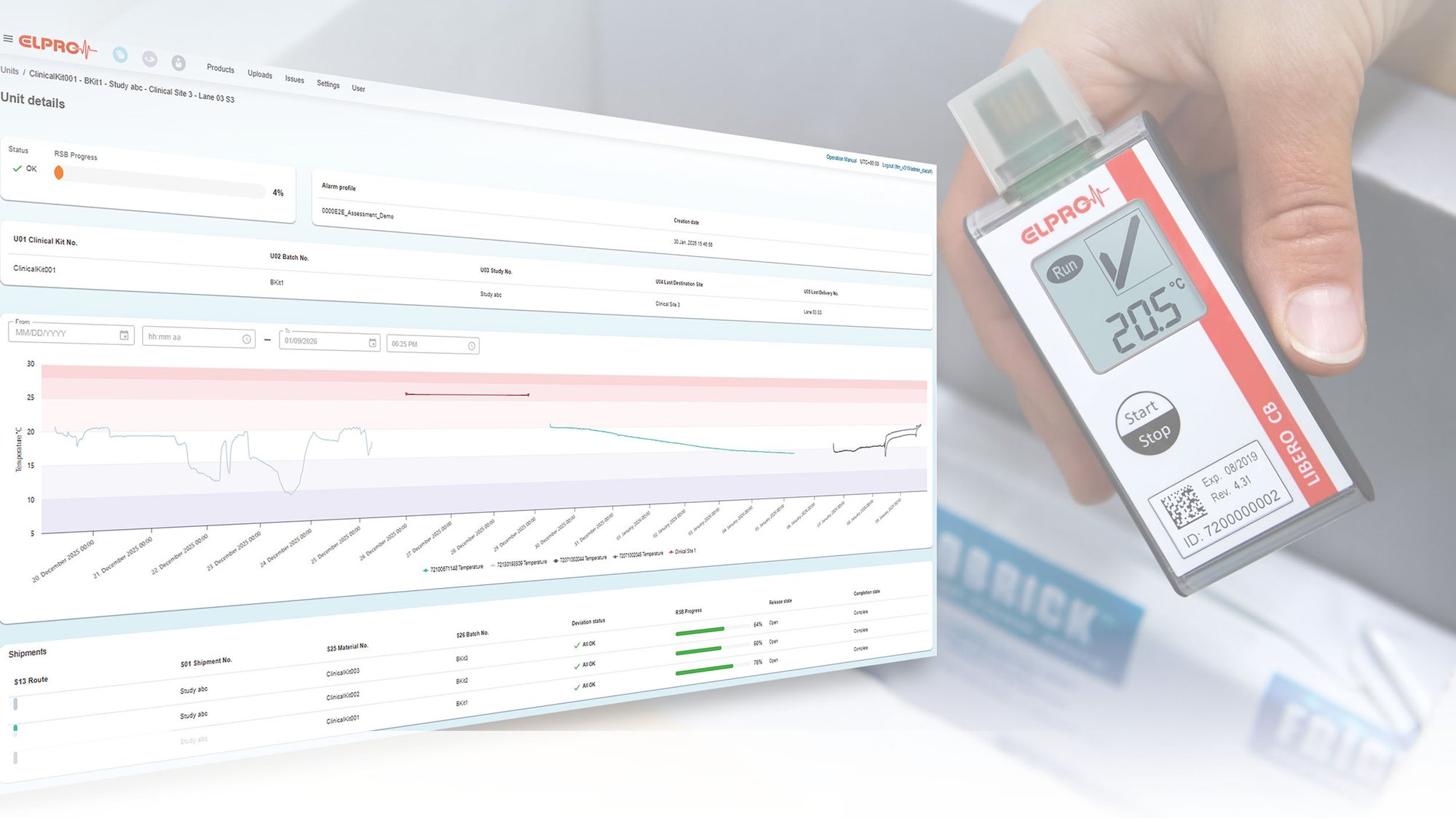
From Production to Clinical Site: End-to-End Monitoring
ELPRO is currently developing a new version of liberoMANAGER that will enable fully kit-based monitoring of clinical supply chains. The goal is to automatically consolidate temperature data, evaluate stability budgets based on defined rules, and make study processes significantly more efficient and transparent.
Key elements:
- Kit-based monitoring across all transport stages – from production to the clinical site.
- Unique kit identifier to automatically link all temperature data throughout the entire supply chain.
- Automated stability evaluation after each transport segment – eliminating manual calculations.
- Integration of external temperature events through manual entries (e.g., intermediate storage, room monitoring systems).
- Automatic release of compliant kits and quarantine classification when thresholds are exceeded.
- Direct integration with IRT/RTSM systems for immediate visibility of status, availability, and releases.
- Reduced manual workload through end-to-end data integration and clearly defined decision rules.
- Optimized interaction between ELPRO hardware and software for reliable and consistent monitoring.
- Greater transparency and traceability across the entire cold chain, combined with enhanced process reliability.