Cold Chain Logistics and its Challenges
All you need to know about implementing the right tools to safeguard your temperature sensitive healthcare products.
Elements of Cold Chain Logistics
There are a number of different supply chains to meet the demands of a pharmaceutical business. They include:
- API Supply: Active Pharmaceutical Ingredients and raw materials needed for manufacturing are shipped inbound to the manufacturing facility in special bulk containers.
- GMP Fill/Finish: A second GMP processing facility to encase drug into final primary packaging.
- Clinical Trial Supply: Investigational Medicinal Products (IMPs) manufactured in small volumes and clinical kits are distributed to hospitals in many different countries.
- Finished Products: After market approval, a product is manufactured in bulk batches and shipped by air, sea or road through several distribution depots often internationally, then locally.
- Last Miles: After a product is sold, it's in the hands of a wholesaler, pharmacy or hospital. This leg is most difficult to track each product. Patient safety is s top concern.
In each supply chain, there are inherent risks depending on your choice of transport mode, packaging equipment and partners.
The Pharma Supply Chain
This supply chain can have up to seven different storage steps and between each “storage step”, the goods need transportation with road, air or ocean. The following illustration shows a simple version of a drug supply chain:
![7_Supply_Chain_Steps[1]](https://www.elpro.com/hs-fs/hubfs/0-LEARN%20(Aioma)/Cold%20Chain%20Logistics/7_Supply_Chain_Steps%5B1%5D.png?width=2937&height=1448&name=7_Supply_Chain_Steps%5B1%5D.png)
Making the Right Cold Chain Logistics and Packaging Choices for your Products
Maintaining temperatures to required specifications during transport is a question of mode (road, air, ocean), size (small quantity of product vs. several pallets), temperature requirements (frozen vs. 2-8 °C strict vs.15-25 °C) and trip length.
Here we review your cold chain logistics choices given by the physical properties, the typical trip lengths and the sizes. The major categories are as follows:
-
Temperature controlled van or truck
-
Active air cargocontainer
-
Passive air cargocontainer
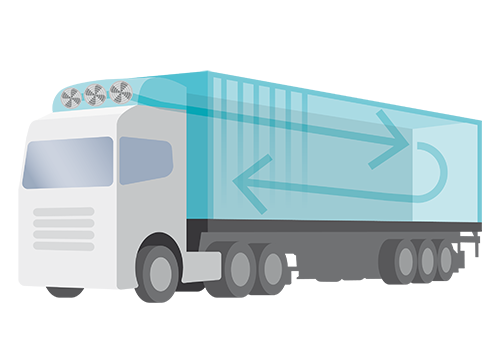
Insulated vehicle, with electrical heating & compressor cooling
- Size: 4-50 pallets
- 2-8 °C
- 15-25 °C (2-25 °C)

Insulated ULD* with electrical heating & compressor cooling
- Size: LD3 - 1 pallet / LD9 - 5 pallets
- 2 - 8 °C
- 15-25 °C (2-25 °C)
- -20 °C
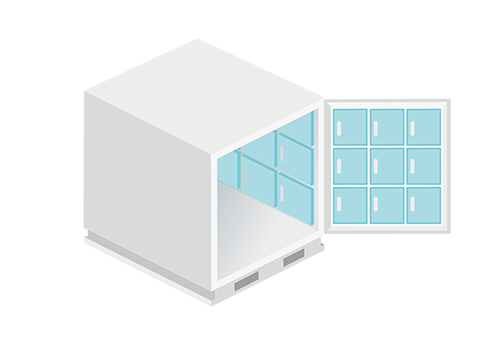
Vacuum insulated ULD* with PCM (phase change material)
- Size: 1 - 5 pallets
- 2 - 8 °C
- 15 - 25 °C (2 - 25 °C)
- -20 °C
-
Insulated Box
-
Ocean reefer container
-
Thermal cover

Vacuum insulated box with PCM (phase change material)
- Various box sizes, 1 - 1000 liter
- 2-8 °C
- 15 - 25 °C (2 - 25 °C)
- -20 °C
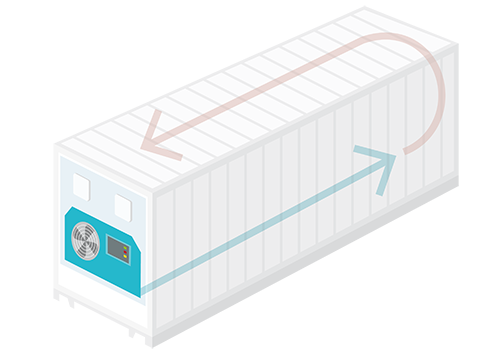
Insulated vehicle, with electrical heating & compressor cooling
- 20 feet: 10-20 pallets
- 40 feet: 20-40 pallets
- 2 - 8 °C
- 15 - 25 °C (2 - 25 °C)
Insulated cover/hood covering the pallet
- 1 pallet
- Light isulation, breaking the peaks
Comparing 5 Cold Chain Risks Using Active or Passive Systems
During a transport, excursions from the promised transport conditions can and will happen. Why?
From experience of millions of pharma shipments analysed in our database, we know that less than 10% of all cases, a true temperature alert (excursion outside defined shipping conditions) is found. Typically, half of the cases are caused by late stop or other mistakes that happen at destination – which could be considered false excursions.
To know the risks, it is important to understand the cold chain logistics equipment:
Wrong Loading
- Active Containers & Trucks (Minimal Risk <0,5%): The equipment must be “fit for use” and powered. Airflow must never be blocked.
- Passive Boxes (Moderate Risk <1%): Passive containers typically require a very particular way to be loaded including many elements such as vacuum insulated panels and bricks containing PCM (phase change material).
Technical Failure During Shipment
- Active Containers & Trucks (Moderate Risk <1%): Active containers/trucks contain hundreds of different mechanical parts that can fail.
- Passive Boxes (No Risk 0%): Passive boxes contain no mechanical parts and therefore during a shipment almost never fail.
Extreme Weather
- Active Containers & Trucks (Medium Risk 1-5%): Extreme weather conditions outside the container/box specification can always happen.
Late Stop
- Passive Containers & Trucks (Medium Risk 1-5%): At destination, site personnel often forget to press the stop button of the data logger.
Handling Errors During Transport
- Active Containers & Trucks (Medium Risk 1-5%): Re-routing, delays, wrong ambient temperature and failure of recharging or not being plugged in.
- Passive Boxes (Medium Risk 1-5%): Re-routing, delays, and wrong ambient temperature (e.g. container left on tarmac for too long).
False Excursions
From experience of millions of pharma shipments analysed in our database, we know that less than 10 % of all cases, a true temperature alert (excursion outside defined shipping conditions) is found.
Typically, half of the cases are caused by late stop or other mistakes that happen at destination – which could be considered false excursions.
Temperature Monitoring of the “API Supply Chain”
Active Pharmaceutical Ingredients (API) are shipped inbound to GMP production facilities from the market authorization holder’s (MAH) facility or another API supplier. APIs come in different forms:
- Liquid ingredients transported frozen, cryogenic or refrigerated
- Lyophilized ingredients transported deep frozen (typically on dry-ice)
- Small molecule powder sensitive to temperature and humidity transported in bags or drums
Typically, large amounts of APIs are shipped with a very high product value. The bags, drums or vessels are then stored until they are combined with excipients (non-medicinal components) to form the final drug. What if during this storage time, temperature went out of specification? Would that Time out of Refrigeration (TOR) be carried forward after the product is manufactured? Probably not. Usually a new stability budget begins at the moment the final product leaves the fill/pack line.
Therefore the challenges of the API cold chain logistics are:
- Special sensitivity of API’s (cryogenic, dry-ice, humidity) requiring special containers logistics means and monitoring solutions
- Challenge of monitoring the drums, bags, containers or vessels during storage. Using an item level monitoring solution (e.g. electronic indicator) might be a solution to carry forward the total time out of refrigeration (TOR) during storage and transport.
Temperature Monitoring of the “Finished Product Supply Chain”
After filling, a drug is in its final primary packaging (blister, vial, respirator or syringe), equipped with all its required leaflets inside a cardboard box, in a sales unit. This sales unit will now start its journey through the supply chain to its final destination in a hospital, doctor’s practice, pharmacy or patient’s home.
But... before it reaches the final destination, it faces the following challenges:
- In a first transportation leg, often large bulk shipments are transported via Truck, Ocean or Air to a distribution depot or regional distributor. Often, one temperature data logger is used for one handling unit containing various different products (with individual stability budgets).
This can cause problems later if there is an alarm in a multi-product shipment – how is each product released? - The second transport leg is often in the hands of a regional organisation which might not have the same access to resources and solutions. Those shipments are often consolidated from different pallets, deliveries or batches.
The problem then is how to carry forward the remaining stability (or the total time out of refrigeration) from the previous transport leg? - In the “last mile”, the challenge becomes even more difficult since sales units get often broken up and the single doses are stored in hospital pharmacies, doctor’s practices or even at home refrigerators. How can the history of the cold chain be re-constructed?
How much total time out of refrigeration (TOR) has the product experienced vs. how much stability budget was initially available?
(Hint: The answer to all these problems... is a cold chain database.)
Where does the responsibility end?
Market Authorization Holders (MAH) have the obligation to guarantee patient safety. But who is responsible for preventing counterfeit and temperature excursions along the entire supply chain? There are numerous risks and the influence of the MAH decreases the deeper we go in the supply chain. 10 years ago, the answer was clear “my responsibility ends, at the moment when the product arrives at my customer (e.g. the local distributor) and the quality release is given”.
However, over the last few years mindsets are changing because:
Investigational Medicinal Products (IMPs) have an increasingly 'closer' supply chain to the patient. Naturally shipping to clinical sites, the shipper is responsible for patient safety, and at the same time ensuring trial efficacy. With more Direct-to-Patient clinical trials, the responsibility is increasing.
Novel biopharmaceuticals present many challenges. Interest is growing to successfully ship these high-value and more sensitive biologics.
In particular new cell therapies, especially autologous therapies, the patient is at the focus of the supply chain.
4 Necessary Data for a Complete Temperature Assessment
If a shipment is complete, a decision needs to be taken to release or quarantine the product. The following data must be available:
- Complete measurement record from a calibrated sensor
- Start flag (clear date and time)
- Stop flag (clear date and time)
- Stability Budget (assessment criteria) with clear conditions of temperature zones/limits and allowed times.
Sometimes additional criteria are defined, like number of allowed excursions or number of freeze-thaw cycles. As soon as all the data is available, the assessment can be performed a clear OK (= release) or ALARM (=quarantine) decision can be taken. Information between stakeholders usually takes place via email.
5 "All to Common" Examples of False Excursions, and How to "Correct" Them
Temperature alarms may not be a final result. Sometimes there are ‘false/positive’ excursions that can be corrected by a cold chain database. Examples of common causes of "false alarms", and their interventions:
- Early start, sensor measures too early (before product has been loaded or conditioned)
Intervention: Cold chain database can re-assess the data using the correct time stamps - No temperature values available due to sensor failure (sensor not started or no sensor added)
Intervention: If the shipment contains more than one device, it might be possible to use this data to release the entire shipment - Minor temperature excursion during shipment
Intervention: Cold chain database can re-assess the data using the stability data of the product - Wrong sensor setting triggers a temperature alarm
Intervention: Cold chain database can re-assess the data using the correct stability data - Late stop at destination, sensor continues to measure (at room temperature)
Intervention: Cold chain database can re-assess the data using the correct time stamps
In any case, it is important to have a “two level” process in place whereby both Logistics and Quality review excursions, before product release.
7 Components of a Cost-Saving CAPAs for Temperature Excursions
In case of a temperature excursion, GMP and GDP regulations require to define a CAPA. A Corrective Action & Preventative Action (CAPA) is a structured process which investigates and identifies root causes of problems and defines corrective action to prevent reoccurrences.
To create a useful CAPA, ask these questions:
- Who found the temperature excursion, when and where?
- What is the scope of the case (shipment number, delivery, handling unit, pallet, product, batch)?
- What is the severity of the excursion?
a. what was the label/transport condition?
b. what was the highest (or lowest) temperature measures?
c. what was the number of excursion hours (can the product still be released based on stability budget? - What was the root cause of the excursion?
- What are Corrective Actions to eliminate this specific problem?
- Have similar cases happened before? Are there patterns in the data?
- Can we define Preventative Actions to make sure similar root causes are eliminated?
For a solid CAPA process in cold chain management, a database is necessary where all data is available in a structured and well documented way.
Is Serialization a Solution for End-to-End Temperature Monitoring?
The problem of counterfeit has received a lot of attention in the past few years. The EU's Falsified Medicines Directive (FMD) is defining serialization as a prerequisite for market authorizations since February 2019. Due to this initiative, each single product unit must carry a unique serial number as standardized barcode referring additional information (such as batch, production date etc.).
![EndtoEndMonitoring_Box_Level_Indicator[1]](https://www.elpro.com/hubfs/0-LEARN%20(Aioma)/Cold%20Chain%20Logistics/EndtoEndMonitoring_Box_Level_Indicator%5B1%5D.png)
During aggregation, the single product boxes are then aggregated to higher levels of packaging (product-box -> sales-unit -> pallet -> delivery unit) again printed as barcodes on the respective level and also captured in a database. A central serialization database – such as tracelink, now knows exactly, which items belong where.
Challenges with Track & Trace
GS1, a non-profit organization that developed and maintains global standards for business communication, even goes a step further and wants to track those aggregated units (e.g. sales-units) along the supply chain and exactly track where the box has been where. If we would in addition also add the respective temperature measurement row into this “global serialization-aggregation-track&trace-database”, we would have the crystal ball and could at any time in the supply chain check this database:
- Is the product in my hand an original (non-counterfeit) product?
- Is the product in my hand o.k. to use (is the total time out of refrigeration this product has experienced so far, inside the defined stability budget)?
The problem with this crystal ball: it is still just vision, and considering all the interfaces that will be needed between all the different ERP-system of each party of the supply chain, implementation will be very complex and result in high cost for each single-product unit.
The Solution - Connecting Serialization with a Box-Level Indicator
Ultimately, we can take two different approaches in addressing the end-to-end monitoring challenge:
- Option A - Measure & Puzzle: Monitor temperatures in the different handling-units, pallets, and boxes following the supply chain and use a database to aggregate all the information.
- Option B – Life-time indicator on box level: Attach an electronic temperature indicator to each product box staying with the product at all time, accounting the total time out of refrigeration (TOR) from the allowed stability budget and indicating if the product is o.k. to use.
By allowing a download on the electronic indicator and connecting to the serialization database, we could even connect the two worlds. On one hand, the serialization database (e.g. tracelink) knows if the product is an original and eventually, the database knows additional aggregation information or even tracking & tracing information. On the other hand, the electronic box-level temperature indicator knows if the product is OK from a perspective of “temperature/stability-budget”. The Smartphone App can now combine both pieces of information “original” & “Temperature = OK” and give a clear result to the user: This drug is safe!
By allowing a download on the electronic indicator and connecting to the serialization database, we could even connect the two worlds:
- The serialization database (e.g. tracelink) knows if the product is an original. Eventually, the database also knows additional aggregation information or even tracking & tracing information.
- The electronic box-level temperature indicator knows if the product is OK from a perspective “temperature/stability-budget”
The Smartphone App can now combine both pieces of information “original” & “Temperature = OK” and give a clear result to the user: This drug is safe!
The Ultimate Dream – A Box Level Real-Time Device
The ultimate dream solving all the mentioned challenges would be:
- A device on box/kit/vial-level staying there for the entire lifecycle (e.g. 3 years), which can measure temperature and manage stability budget end-to-end, and transmit this data wirelessly to a cloud database (therefore also knows at all times where it is geographically).
- Then the cloud database would alarm in case of deviation, know whose fault it was, even prevent deviations by pro-active interventions, and it could further automate release at each handover-point, and even automatically manage stock at the hospitals/pharmacies.
![Ultimate_Dream_Real-Time_Dataloggers[1]](https://www.elpro.com/hubfs/0-LEARN%20(Aioma)/Cold%20Chain%20Logistics/Ultimate_Dream_Real-Time_Dataloggers%5B1%5D.png)
Disney said, “If you can dream it, you can do it”. But unfortunately today a box level real-time device is technically not possible:
- Although Mobile-IoT networks are developing fast, the communication chips as well as the roaming cost are still too expensive to be applied on a chip level
- The Mobile-IoT communication chips still need a large amount of energy to communicate with nearby public antenna networks. If we would try to cover a lifespan of 2+ years, we would need a huge battery pack
- And such a device would never fit on box/kit/vial-level (LPWAN communication chip, Mobile-IoT antenna, large battery-pack)
What we see is an increasing discussion around gateway concepts. So a larger unit (e.g. box) is equipped with an IoT-gateway which is communicating via Bluetooth to mini-sensors sitting on vials in the same box.
Let's Talk Cold Chain Distribution. Contact Us Today.

Newsletter-Sign Up ELPRO News
ELPRO will use the information provided in this form to keep in touch with you and to send you updates and marketing information by e-mail.
