Rooms & Equipment Monitoring Solutions
Compliant, Scalable Solutions for Monitoring Production, Storage and Equipment
Highly regulated environments and laboratory equipment in the pharmaceutical, life sciences, and healthcare industries demand precise monitoring to ensure compliance. Whether it's refrigerators, ultra-low freezers, incubators, cryogenic (LN2) tanks, cold storage rooms, cleanrooms, production sites, or global warehouse networks—ELPRO offers trusted solutions for virtually any application.
- Modular, customizable systems with wired, wireless, and IoT devices for all critical parameters
- Rapid adaptation to industry standards and evolving requirements
- Built-in transparency, data integrity, and regulatory compliance
- Protection of assets and robust data security
- Reduced product loss and improved operational efficiency
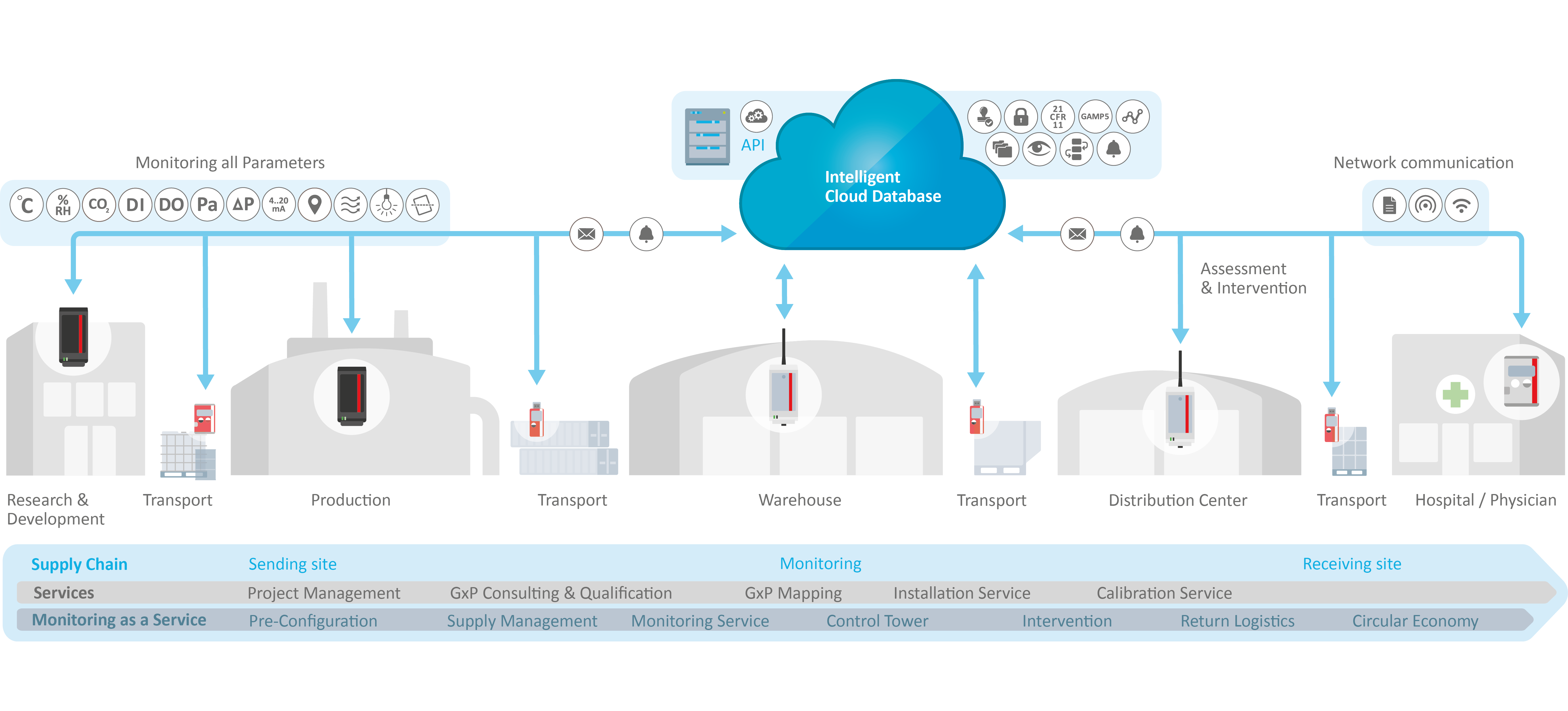
Scalable, Integrated Solution for Various Parameters
- Modular, customizable solutions with wired/wireless sensors for all critical parameters
- Available as an on-premise and a software-as-a-service solution
- Intuitive software (24/7 access) with clear data visualization
- Integration into existing user management (Active Directory or Single Sign-On)
- Management of multiple locations via one solution
- Immidiate alarming and notifications
- Automated reporting and analyses
- API integration for seamless data transfer to other systems

Cloud-Based Real-Time Monitoring Made Easy
- Radio modules and IoT data loggers to monitor rooms and equipment
- Global 24/7 access
- Easy to set up, modify and expand
- Immediate alerts via e-mail and/or SMS
- FDA 21 CFR Part 11 compliant and GAMP® 5 validated
- Unlimited number of sensors
- API for integration into your system (optional)
Comprehensive Portfolio of Devices for Superior Connected Performance
Sensors and data loggers to monitor almost every parameter needed—temperature, humidity, differential pressure, particles, and more.

Wired Modules
- °C, %RH, 4..20mA, DI, DO
- Stable penetration into shielded rooms
- Unlimited number of sensors
- R&D labs/cleanrooms
- Personalized medicine labs
- Biotech start-up centers
- Research universities/hospitals

Wireless Modules
- °C, %RH, 4..20mA
- Radio frequency: 868 / 915 MHz
- Flexible positioning of sensors
- Up to 50 sensors per local radio bridge
- R&D labs & cleanroom
- Storage facilities & cold rooms
- Clinical sites

Cellular IoT Real-Time
- °C, %RH
- LTE-M and NB-IoT (global roaming)
- Scalable IT-independent installation
- Stationary cryo & LN2 shippers
- Refrigerators, (ULT) freezers
- Storage rooms & warehouses
- Clinical shipments and storage
ELPRO system notifications play a key role in emergency resilience. With potential losses of $50,000 per incident, ELPRO's implementation leads to significant annual savings and a quick return on investment.
elproMONITOR provides us a key aspect of resilience for temperature monitoring.
Compliance Made Easier
Navigating global regulations and standards can be complex—but with expertise, it doesn’t have to be. Comprehensive solutions are provided to keep companies compliant and prepared for upcoming audits, ensuring smooth operations worldwide at all times. The complexity is handled, allowing focus on what matters most—your success.

Let's Talk Room & Equipment Monitoring

Frequently Asked Questions About Monitoring Rooms & Lab Equipment
Does the solution protect data integrity and meet regulatory compliance requirements?
Does the solution provide real-time monitoring and alert mechanisms?
Environmental monitoring solutions must offer robust real-time monitoring and notification capabilities to effectively manage all critical storage environments, ensuring the safety and integrity of sensitive products. These solutions provide continuous, automated temperature and environmental parameter tracking, crucial for immediately detecting out-of-specification (OOS) conditions that could compromise product quality. Utilizing these advanced systems, alerts are dispatched instantly via SMS, email, or mobile app to relevant personnel, enabling prompt corrective action. The integration of multi-tier escalation protocols ensures that notifications are channeled through appropriate levels of management based on severity, reinforcing swift decision-making under pressing circumstances. Furthermore, seamless integration with existing software-based alarm management systems allows for centralized control and coordination, enhancing response efficacy. These systems can monitor diverse environments such as ultra-low temperature freezers, liquid nitrogen storage, cleanrooms, production areas, and warehouses, all of which require meticulous oversight to maintain compliance with stringent regulatory standards. By prioritizing real-time capabilities, organizations can safeguard their products against environmental threats, preserving both quality and regulatory compliance.
Does the solution have reliable and calibrated sensor and probe technology
When examining the technical specifications and accuracy of monitoring sensors, particularly for critical storage conditions such as those required for cell and gene therapies, it is essential to prioritize precision and reliability. A fundamental consideration is the precision of temperature measurement, where an accuracy of ±0.1 °C is often necessary to ensure the integrity of these sensitive biological materials. Employing wireless sensor technologies can enhance flexibility in monitoring setups, reducing clutter and facilitating easier data retrieval as opposed to wired counterparts. Battery life and replacement protocols are critical factors, as extended battery life and easy replacement processes minimize maintenance interruptions and ensure continuous monitoring. Calibration traceability is indispensable to confirm that sensors consistently deliver accurate readings over time, a crucial aspect for maintaining compliance and data validity. It's important to incorporate redundant sensor systems in critical storage locations to act as fail-safes, mitigating the risk of data gaps due to sensor malfunctions. Additionally, sensors must perform reliably across a wide range of temperatures, from extreme lows of -196 °C to highs of +37 °C, to accommodate the diverse storage needs typical in biopharmaceutical environments.
How robust are the data management and reporting capabilities?
Efficient data management is crucial for environmental monitoring, especially in pharmaceutical and laboratory settings where precision and compliance are vital. Important features include cloud-based and local data storage for redundancy and security, automated report generation for timely analysis, and long-term data archiving for compliance. Access to data in formats like PDF and CSV allows sharing and integration into other tools. A comprehensive dashboard aids quick visual monitoring, while trend analysis tools offer insights into environmental conditions. Integration with systems like LIMS or ERP ensures seamless data flow, and advanced user group management customizes access and permissions.
Does the solution offer scalability and enterprise integration potential?
In evaluating the flexibility of an environmental monitoring solution for future growth and integration with existing laboratory information management systems (LIMS), there are several key considerations to address. First, you must decide between cloud-based and on-premise solutions. Cloud-based systems offer scalability and ease of access, which are ideal for organizations expecting rapid growth or those operating across multiple locations. On the other hand, on-premise solutions can offer heightened security and offline accessibility, crucial for protecting sensitive data. Another important factor is API connectivity, which ensures seamless data exchange and communication with existing systems, enhancing overall operational efficiency. Compatibility with major equipment manufacturers is also essential to future-proof your system and ensure it can adapt to advancements in technology or changes in laboratory workflows. Furthermore, evaluate the ease of software updates and maintenance protocols, as a well-supported system can reduce downtimes and provide uninterrupted service. Finally, consider the long-term costs associated with scaling the solution. A cost-effective model allows for strategic growth while ensuring that investments align with the organization's budgetary constraints and expansion goals.
Does the solution offer compliant validation and qualification documentation?
When selecting an environmental monitoring solution, it is imperative to evaluate the level of validation documentation and support the vendor provides in facilitating regulatory compliance. Ensuring that the vendor supplies robust IQ/OQ/PQ (Installation, Operational, Performance Qualification) documentation is crucial, as these documents confirm that the system is designed, installed, and performs according to specified requirements. Additionally, the vendor's track record with regulatory inspections should be scrutinized, as a strong history reflects their capability to meet compliance standards consistently. Comprehensive technical support for audit preparation is essential, offering confidence that your organization can handle regulatory inquiries efficiently. Furthermore, the availability of detailed training resources ensures that your team can effectively operate and maintain compliance with the system. Vendors should also provide well-documented risk management processes, demonstrating their commitment to maintaining system integrity under various conditions. Lastly, proof of compliance with current Good Manufacturing Practices (cGMP) solidifies the vendor's dedication to ensuring both quality and regulation adherence throughout the operational lifecycle.
What is the total cost of ownership (TOC)?
Understanding the total cost of ownership (TOC) requires a holistic view that extends beyond the upfront Capex investments in software and hardware. This comprehensive approach should include the costs associated with operational training to ensure personnel are well-prepared to utilize the system efficiently, reducing the learning curve and potential for errors. Additionally, support services, both during initial deployment and throughout the system's lifecycle, must be factored in to ensure sustained operational reliability. Regular calibration, maintenance and timely upgrades are critical components of this ongoing commitment, necessary for adapting to technological advancements and regulatory shifts. Moreover, the cost analysis should incorporate considerations of scalability, as the ability to expand without requiring complete overhauls contributes to cost-effectiveness over time. By closely monitoring the mean time between failures (MTBF), organizations can anticipate maintenance needs and proactively manage system downtime, thereby minimizing disruptions and ensuring continuous operational efficiency.
Other critical considerations
- Global technical support availability is essential for addressing issues promptly and minimizing disruptions, especially for multinational operations with a broad geographic scope.
- Strong cybersecurity protections must be in place to safeguard sensitive data against unauthorized access, integrating the latest encryption protocols and threat detection technologies.
- Lastly, the solution should demonstrate robust capabilities for continuous monitoring during power outages or network disruptions, ensuring data integrity and security through resilient design features like backup power supplies and autonomous data logging functions.
Newsletter-Sign Up ELPRO News
ELPRO will use the information provided in this form to keep in touch with you and to send you updates and marketing information by e-mail.


