Cold Chain Logistics
Environmental Monitoring in Pharma Cold Chain Logistics
As the cold chain ecosystems evolve, validated monitoring solutions throughout the distribution process play a vital role. Cold chain monitoring refers to the continuous measurement, documentation, and control of temperature and environmental conditions to protect temperature-sensitive pharmaceutical products during storage, transport, and distribution.
To ensure product quality and regulatory readiness, modern cold chain monitoring now includes real‑time visibility, digital automation, and end‑to‑end data integrity across global distribution networks. Maintain the integrity of your temperature-sensitive pharmaceuticals, vaccines, precision medicines, tissue samples, and medical devices with:
- Real-time continuous precision monitoring
- Wide range of parameters (temperature, humidity, light, tilt, and position)
- Comprehensive hardware, software and service solutions for global regulatory compliance
- Auto-assessment and software-based alarming for accelerated assessments and product releases
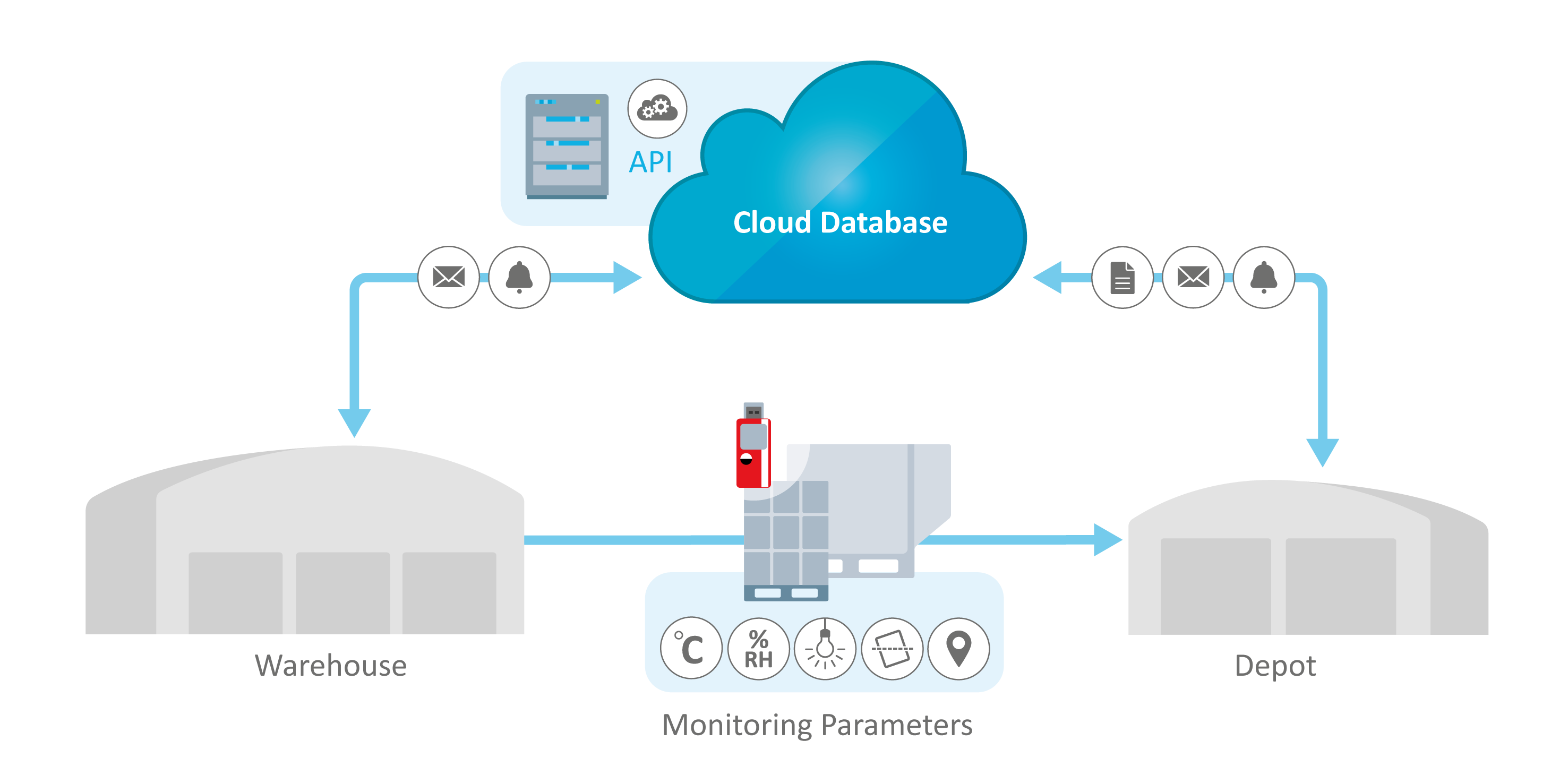
What Is Cold Chain Monitoring?
Cold chain monitoring is the process of continuously tracking and recording temperature and environmental conditions throughout the pharmaceutical supply chain. Using validated temperature data loggers, real-time IoT sensors, and compliant monitoring software, organizations can verify that pharmaceutical products remain within approved temperature ranges.
This monitoring provides documented proof that storage and transportation conditions meet GDP (Good Distribution Practice), GMP (Good Manufacturing Practice), and regulatory requirements such as FDA 21 CFR Part 11. Cold chain monitoring protects product stability, supports regulatory audits, and ensures patient safety by maintaining product integrity throughout the supply chain lifecycle.
History of Cold Chain Monitoring
Cold chain monitoring has evolved significantly alongside pharmaceutical innovation. Early cold chain logistics relied on manual temperature checks and mechanical recording devices, which provided limited visibility and delayed deviation detection. With the rise of biologics, vaccines, and personalized medicine, more advanced monitoring technologies became essential to ensure temperature stability and regulatory compliance.
Today’s cold chain monitoring solutions incorporate digital temperature data loggers, wireless IoT sensors, cloud-based monitoring platforms, and automated alarming systems. These modern systems provide real-time visibility, automated compliance documentation, and predictive insights to optimize supply chain performance and reduce product loss.
From cold chain monitoring to temperature-controlled logistics
Cold chain monitoring has expanded beyond simple temperature recording into fully integrated temperature-controlled logistics. Modern pharmaceutical supply chains use intelligent monitoring systems to optimize shipping routes, qualify thermal packaging, and maintain continuous visibility from manufacturing to patient delivery.
These systems enable proactive intervention during transport, improve shipment performance analysis, and support advanced logistics strategies such as mixed-load shipments, direct-to-patient delivery, and global clinical trial distribution.
Cold Chain Pharmaceutical Logistics Products Need Protection
For over three decades, ELPRO has built a deep understanding of your cold supply chains, how to mitigate risk within them, and how to protect product efficacy, process efficiency and compliance throughout the entire journey. Cold chain logistics ensures that temperature-sensitive pharmaceutical products remain within validated temperature ranges—from manufacturing and storage to transportation and final delivery—preserving safety, efficacy and regulatory compliance.
-
Commercial
-
Clinical Trials
-
Precision/Personalized Medicine

Commercial Pharma Cold Chain Monitoring
- Monitoring of validated cold storage facilities (-80 °C to +25 °C) with backup systems
- Continuous environmental monitoring during transport
- Monitoring of thermal packaging qualified for specific temperature ranges
- Real-time deviation alerts with intervention protocols
- Temperature verification documentation
These cold chain monitoring solutions ensure pharmaceutical manufacturers maintain full regulatory compliance while protecting high-value drug products, vaccines, and biologics during storage, transportation and global distribution.

Clinical Trials Pharma Monitoring
- Continuous monitoring with validated devices
- Qualified monitoring of thermal packaging
- Temperature mapping services for storages and equipment
- Validated shipping routes with minimal handoffs
- Real-time temperature tracking with excursion alerts
Clinical trial cold chain monitoring is essential to ensure investigational medicinal products remain within validated temperature ranges. Monitoring protects sensitive biologics, cell and gene therapies, and clinical samples from temperature excursions that could compromise trial validity or patient safety.
Advanced monitoring solutions provide real-time shipment visibility, enabling immediate corrective actions, reducing risk, and ensuring regulatory compliance throughout global clinical trial supply chains.
Precision Medicine Cold Chain
- Validated continuous monitoring devices meeting regulatory standards
- Qualified monitoring of thermal packaging designed for sensitive personalized treatments
- Temperature mapping services for storages and equipment
- Data for optimized shipping routes minimizing handoffs for irreplaceable samples
- Real-time alerts that enable rapid interventions instead of retrospective assessments
Precision medicine cold chains require the highest level of temperature control, as therapies such as CAR-T cells, gene therapies and biologics are often irreplaceable and highly sensitive to environmental conditions. Continuous monitoring ensures patient-specific treatments maintain viability from manufacturing to administration.
Smart Solutions for Complex Supply Chains
Hardware and software solutions from ELPRO help maintain quality and compliance throughout the pharmaceutical and life science supply chain. Designed to simplify complex processes, they support faster, well-informed decision-making—from production to delivery.
- Data loggers for every pharma application
- Intuitive software with clear data visualization
- Customizable workflows
- Advanced user / access management
- Real-time alarms and notifications
- Automated reporting
- Auto assessments and software-based alarming for mixed-load shipments
- API integration for seamless data transfer to other systems


Single-Use USB
- Temperature
- Automated PDF report generation
- USB connection
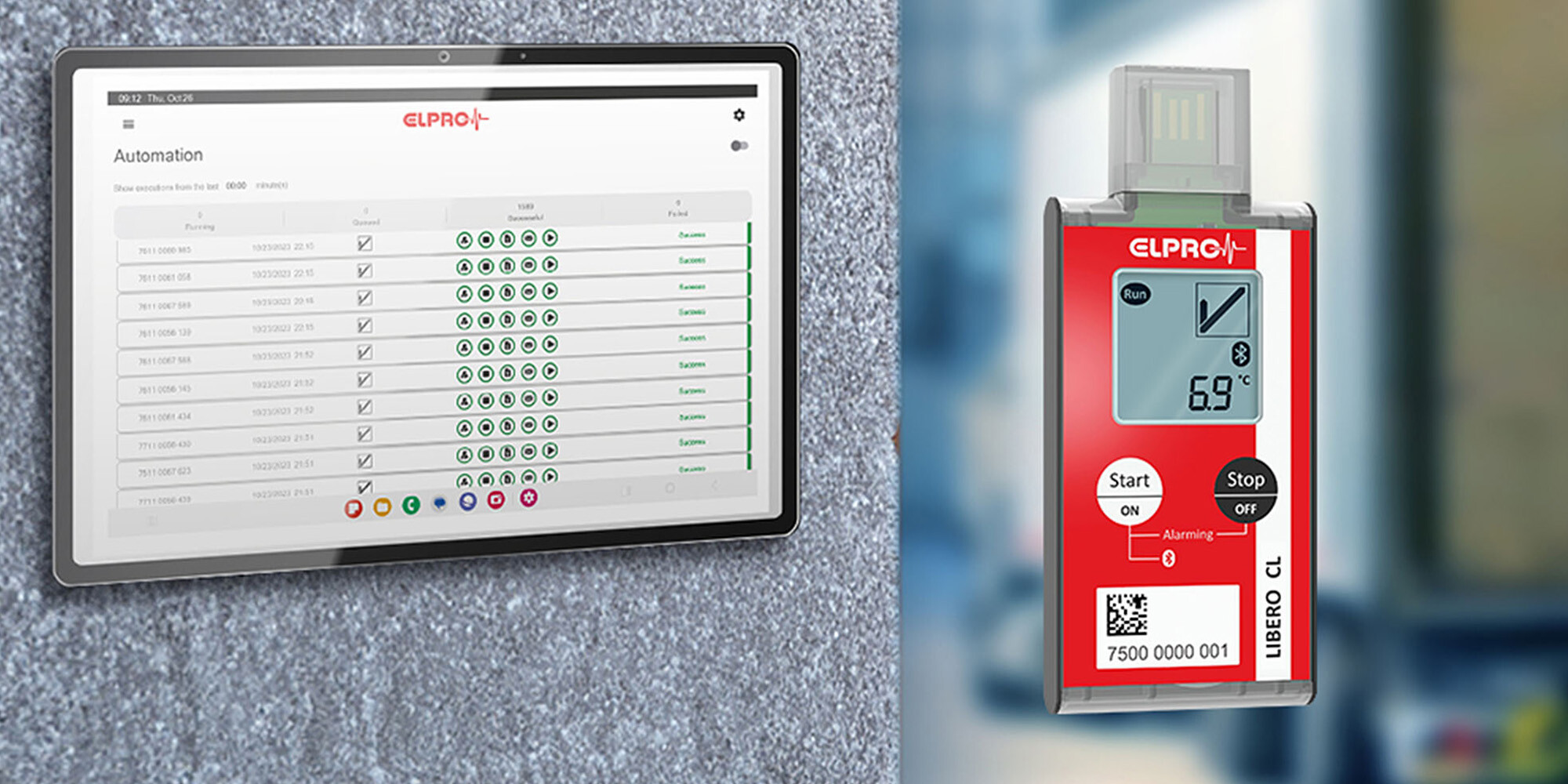
Multi-Use Bluetooth®
- Temperature and humidity
- USB or Bluetooth® connection
- Automated read-out of data or PDF

Cellular IoT Real-Time
- Temperature, humidity and position
- Real-time data generation and alarming
- Single-Use / Multi-Use
Monitoring Solutions for Every Temperature Range
Ambient
+15 °C to +25 °C
Used for standard pharmaceutical tablets, capsules and suitable for stable diagnostic kits; protects temperature-sensitive medical devices; also used for cosmetics requiring consistent conditions
Cool
+2 °C to +8 °C
Common in pharmaceuticals, vaccines, biologics, and injectable drugs like insulin; essential for gene/cell therapies; preserves supplements and probiotics; maintains diagnostic kit stability
Frozen
-20 °C to -80 °C
Essential for gene therapies, biologics, and viral vectors; preserves cell/tissue and stem cells; required for certain vaccines needing deep freeze; used for cryopreservation of biological materials
Dry Ice
-40 °C to -80 °C
Used for biologics and gene therapies; preserves viral vectors and cell-based treatments; critical for mRNA vaccines; maintains cell lines and various highly-sensitive biological samples
Cryogenic
-150 °C to -196 °C
Effectively suspends biological activity, prevents degradation and preserves biological function for extended periods. Protects cell/gene therapies, stem/CAR-T cells, tissue samples and more
Comparing our old process to the new one is like comparing a bike to a car. Both can cover a certain distance, but in terms of speed and comfort, they are incomparable. The new solution stands for long-term modernization, digitalization and entry into the modern world.
Compliance Made Easier
Navigating global regulations and standards can be complex—but with expertise, it doesn’t have to be. Comprehensive solutions are provided to keep companies compliant and prepared for upcoming audits, ensuring smooth operations worldwide at all times. The complexity is handled, allowing focus on what matters most—your success.

Let's Talk Cold Chain Distribution. Contact Us Today.

Common Questions About Cold Chain Logistics
Regardless of the nature of your cold chain logistics application, you demand a reliable, compliant environmental monitoring solution that will scale and grow with your organization, protect your valuable products and ensure quality and data integrity from end-to-end. Below is a list of important questions ELPRO recommends you ask service providers when evaluating a solution.Why is cold chain monitoring critical for clinical trials?
Clinical trials rely on temperature-sensitive investigational products, biologics, and samples that must remain within strict temperature ranges. Cold chain monitoring ensures data integrity, regulatory compliance, and patient safety by providing documented proof of proper storage and transport conditions.
Continuous monitoring reduces risk, prevents product loss, and ensures trial validity across global clinical trial supply chains.
What is cold chain distribution in a supply chain?
There are many complex temperature monitoring scenarios involved when considering cold chain distribution in a supply chain. Several temperature-controlled scenarios for global delivery below may include the following data logger or monitoring device type:
- One way with product (single-use)
- Multi-step cold chain depots (multi-use)
- One way on product and cold room storage (single-use)
- End-to-end on product (box-level temperature monitoring indicators)
- Sample collection (box-level temperature monitoring indicators)
- One way with product and return (single-use or electronic temperature indicators)
- Continuous on mobile equipment (real-time on containers and/or transport vehicles)
What are supply chain qualification requirements?
For cold chain products and equipment used in transit, anything used in a GDP or GMP environment to store or transport products must be qualified. This applies to containers, cold boxes, airplanes, truck and vans fleets, and even trade lanes. Qualification is the process of proving that a piece of equipment, a room, a shipping lane, or even an entire network fulfils an intended purpose. Compared to qualifying equipment such as cryogenic shippers or freezers, and rooms, the qualification of an entire network is much more complex and requires significant analysis and process documentation.
Qualification ensures temperature-controlled logistics equipment, storage environments, shipping containers and transport routes consistently maintain validated environmental conditions required to protect pharmaceutical product quality.
Does the solution ensure real-time monitoring and alerting of temperature excursions?
If your application requires continuous monitoring, be certain to determine, if the IoT sensors continuously monitor all parameters and transmit data in real time via e-mail, SMS, or app notifications when a temperature deviation is detected, ensuring more time for rapid corrective action.
Real-time cold chain monitoring enables immediate corrective action, reducing product loss and ensuring regulatory compliance throughout transportation and storage.
Is a environmental monitoring system compliant with GDP and GMP regulations?
If you require an environmental monitoring system, your solution should comply with GDP, GMP, be GAMP5 validated and comply with 21 CFR Part 11. It ensures audit-ready data integrity and proper temperature control to maintain regulatory compliance. Compliance ensures complete data integrity, audit readiness, and regulatory acceptance for pharmaceutical cold chain monitoring.
How is data integrity maintained, and does the system offer tamper-proof or auditable records?
The system must employ encrypted data transmission and secure cloud storage to ensure data integrity. It must also feature automated audit trails, so all system activities are logged and can be reviewed for compliance purposes.
Can the solution provide end-to-end visibility for temperature control across the entire cold chain (from manufacturing to transportation to storage)?
Intelligent database solutions such as ELPRO's liberoMANAGER and elproMONITOR provide comprehensive end-to-end visibility through cloud-based dashboards, offering real-time tracking of environmental parameters at every stage of the cold chain, whether in transit, at storage facilities, or during manufacturing.
What is the scalability and flexibility of the system as our needs grow or change?
The system you select should be highly scalable, capable of supporting additional sensors, warehouses, and geographies as your operations grow and expand, even globally. Be sure it can be configured to monitor multiple environmental parameters and integrate with other enterprise (ERP) business systems as your operations expand.
How does the system handle data logging and reporting, and can it generate customized compliance reports?
All data need to be logged securely and continuously. The system must be able to generate fully customized reports for compliance audits. Reports could include temperature history, excursion details, and corrective actions, formatted to meet regulatory requirements.
What backup or contingency measures are in place in case of system failure or connectivity issues?
In the event of system failure or lost connectivity, sensors and hardware need to buffer/store data locally and automatically sync with the cloud once connection is restored. Your system should also have redundant servers to prevent critical data loss and ensure continuity.
Does the system support integration with other enterprise software (e.g., warehouse management, transportation management, ERP systems)?
Environmental monitoring database solutions like elproMONITOR and ELPRO's liberoMANAGER are designed to integrate seamlessly with various enterprise platforms, allowing for centralized control and dashboard visibility across the entire supply chain.
What are the system’s capabilities for monitoring multiple environmental factors beyond temperature (e.g., humidity, light exposure, vibration)?
The cold chain logistics database solutions must support multi-parameter monitoring, including temperature, humidity, light exposure, tilt, vibration, with customizable alerts for each environmental factor to ensure comprehensive protection of valuable and sensitive products.
How does the solution handle the validation and calibration of sensors and devices?
The system must be validated and calibrated to meet the specified quality and ISO standards in your SOP. Calibration certificates and routine inspections ensure that hardware and sensors remain accurate and compliant with industry standards.
What is the system's user-friendliness, and how much training is required for staff to operate it effectively?
The system design and interface should be intuitive and user-friendly, with a simple features and dashboards for monitoring, conducting analyses, assessments and generating reports and archiving.
What is the expected response time and customer support availability in case of issues?
Customer support should be made available 24/7, offering rapid response times. Issues should typically be addressed within hours, via phone, email, text and online chat support. The environmental monitoring system should also feature remote alarming capabilities to immediately notify stakeholders of deviations via e-mail or SMS. The feature should also enable system manager to assign user groups to ensure redundancy.
What is the total cost of ownership (TOC), including installation, maintenance, and any hidden or recurring fees?
The initial cost of an intelligent cold chain logistics database and environmental monitoring solution includes hardware (sensors and data loggers), software, installation/setup. Optional costs may include onboard system training, project management and GxP services (GDP/GMP, qualification and temperature mapping, for example) to ensure compliance, if required. Ongoing costs are typically subscription-based software access, cloud storage, and maintenance. A detailed quote should outline all associated fees and services.
How does the system support GDP/GMP temperature mapping and qualification for storage and transportation facilities and equipment?
The cold chain logistics temperature monitoring solution should include services such as temperature mapping to document variability in specific storage or transport areas. It should also support qualification reports to demonstrate compliance with regulatory guidelines during facility audits.
Newsletter-Sign Up ELPRO News
ELPRO will use the information provided in this form to keep in touch with you and to send you updates and marketing information by e-mail.


