ECOLOG-PRO
Environmental Monitoring in Production and Cleanroom
Pharmaceutical cleanrooms and production areas are highly sensitive zones where even slight changes in temperature, humidity, or pressure can impact product quality and patient safety. With unpackaged products exposed, contamination risks are high. Protecting product integrity and personnel requires monitoring solutions that cover:
- Continuous monitoring and recording of all relevant parameters
- Immediate alarms through clearly defined channels
- Regulatory-compliant system qualification and documentation
- Clear data visualization, analysis tools, and automated reporting
- Scalable system design for future production expansion
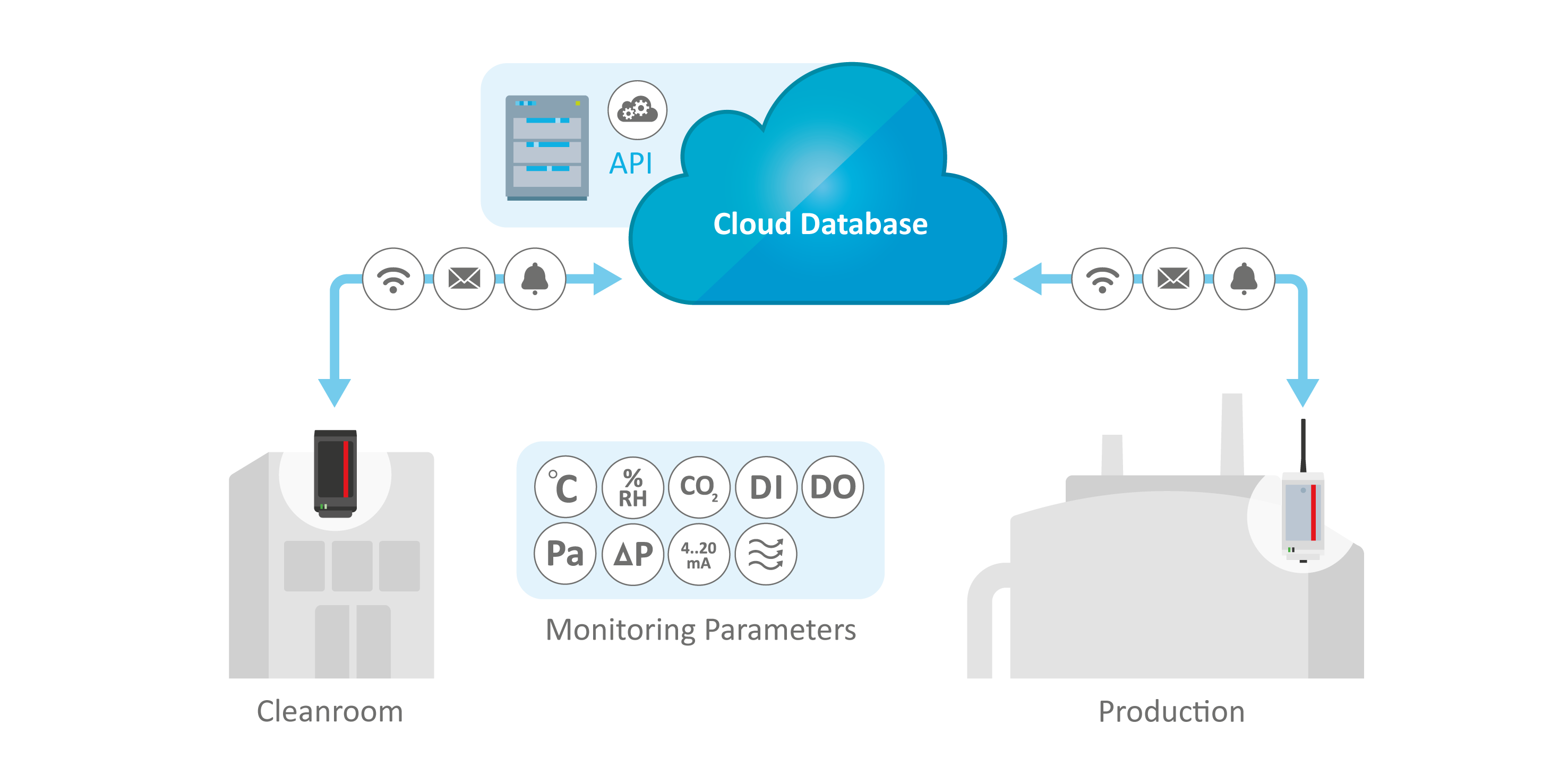
Relevant Parameters Seamlessly Monitored and Documented
-
Every Parameter
-
Enterprise Integration
-
Global Compliance

Advanced Monitoring Control
- Covering all critical parameters such as temperature, relative humidity, particle count, (differential) pressure, air flow, carbon dioxide levels, door openings
- Integration of signals from additional hardware possible
- Audible and visual alarms in the room, in the corridors or via flexible displays
- Sensors calibrated to the ISO 17025 standard

From Basic to Complex Multi-Site Installations
- Wired and wireless options adapt to today's modular manufacturing facilities
- Connected systems that integrate easily into existing infrastructure, supporting both small and large facilities
- Unlimited sensor connections

Audit-Ready Implementation
ELPRO solutions meet stringent GMP/GDP, ensuring superior data integrity for all your regulated environments.
- GAMP® 5 validated
- FDA 21 CFR Part 210/211 (USA)
- EU GMP Annex 1 (Europe)
- PIC/S GMP Guide (international)
- ISO 17025 calibration
- ISO 14644 (international)
- ICH Q7 (international)
Scalable, Integrated Solution for Various Parameters
Hardware and software solutions from ELPRO support reliable monitoring of pharma and life-sciences cleanrooms and production areas. Designed to meet strict regulatory requirements, they ensure consistent environmental control and enable faster, more informed decision.
- Modular, customizable solutions with wired/wireless sensors for all critical parameters
- Intuitive software (24/7 access) with clear data visualization
- Integration into existing user management (Active Directory)
- Management of multiple locations via one solution
- Immidiate alarming and notifications
- Automated reporting and analyses
- API integration for seamless data transfer to other systems


Wired Modules
- °C, %RH, 4to20mA, DI, DO
- Wired
- Stable penetration into shielded rooms
- Unlimited number of sensors

Wireless Modules
- °C, %RH, 4to20mA
- Radio frequency: 868 / 915 MHz
- Flexible positioning of sensors
- Up to 50 sensors per local radio bridge
From GxP Consulting and Services to a Cleanroom Monitoring System
Read about this US biotech startup relying on ELPRO’s GxP services and consulting to successfully implement easy-to-use
environmental monitoring system for five clean rooms, -80 °C freezers, refrigerators, incubators, and a warehouse.
Our biotech customer appreciated and valued the ELPRO project management, our on-site GxP service and consulting. They were immediately convinced by the ease of use of our software.
Compliance Made Easier
Navigating global regulations and standards can be complex—but with expertise, it doesn’t have to be. Comprehensive solutions are provided to keep companies compliant and prepared for upcoming audits, ensuring smooth operations worldwide at all times. The complexity is handled, allowing focus on what matters most—your success.
- Project Management
- GxP Consulting and Qualification
- GxP Mapping Service
- On-/Off-Site Services
- Monitoring as a Service

Contact Our Cleanroom and Production Consultants Today

Common Questions To Ask About Monitoring Cleanrooms and Production Facilities:
Highly regulated environments such as cleanrooms and manufacturing production facilities at biotech, life science, and pharmaceutical companies, require critical control for numerous parameters. Below is a list critical questions to consider when evaluating critical solutions for complient environmental control:What is the Total Cost of Ownership (TCO)?
Formulate a detailed breakdown of all costs including:
- Initial hardware procurement costs
- Server and software licensing (perpetual vs. subscription)
- Installation and initial validation expenses
- Annual maintenance and support fees
- Sensor replacement and calibration costs
- Training and knowledge transfer expenses
- Potential upgrade paths and future compatibility
- Hidden costs (infrastructure modifications, additional integrations)
Can I Ensure Data Integrity and Regulatory Compliance?
- How does your solution ensure 21 CFR Part 11 compliance for electronic records and signatures?
- Provide detailed documentation on your system's audit trail capabilities, including timestamping, user authentication, and change tracking.
- What built-in features prevent data manipulation and ensure complete traceability for regulatory inspections?
Are Validation Documentation and Audit Trails Included?
Comprehensive validation documentation should include:
- Installation Qualification (IQ) documentation
- Operational Qualification (OQ) protocols
- Performance Qualification (PQ) templates
- Risk assessment documentation
- Detailed test scripts and acceptance criteria
- Validation Standard Operating Procedures (SOPs)
- Traceability matrix linking system requirements to validation evidence
What Real-Time Monitoring and Alerting Capabilities Are Included?
Ask important questions such as:
- Describe your system's real-time monitoring capabilities across multiple parameters (CO2, O2, temperature, particle count, differential pressure, relative humidity).
- What are the alert mechanisms? Can the system provide:
- Instantaneous notifications via multiple channels (email, SMS, mobile app)
- Escalation protocols for critical threshold breaches
- Customizable alert thresholds for different facility zones
- What is the system's response time from detection of an out-of-specification condition to alert generation?
Do I Require Data Integration and System Interoperability?
- How does the solution integrate with existing facility management systems, LIMS, and other enterprise software?
- What are the supported data exchange protocols (HL7, REST API, etc.)?
- Can the system export encrypted data in formats required by regulatory agencies (PDF, CSV, XML)?
- Describe the software validation support package for ensuring system compatibility and compliance during implementation.
How Reliable Are Sensor and Hardware Solutions?
- Get detailed specifications on sensor accuracy, calibration requirements, and long-term stability
- What is the mean time between failures (MTBF) for your sensors and monitoring equipment?
- Describe your sensor redundancy and failsafe mechanisms
- What are the recommended calibration and maintenance intervals?
- How does the system handle sensor failures or data collection interruptions?
Is Cloud and Cybersecurity Infrastructure Is Required?
- If seeking a cloud-based solution, get the cloud infrastructure's security certifications (e.g., ISO 27001, SOC 2)
- Determine what encryption protocols are used for data at rest and in transit?
- Ask about managing access control, including multi-factor authentication and role-based permissions?
- Determine details on your disaster recovery and business continuity plans
- Determine your batch management and vulnerability monitoring process
Are References on Implementations at Similar Facilities Available?
Seek references that demonstrate:
- Successful implementation in FDA-regulated environments
- Experience in specific subsectors (e.g., biologics, small molecule manufacturing, cell therapy)
Documented performance in:- Data integrity maintenance
- Regulatory audit success
- Minimal system downtime
- Effective support during critical incidents
Request:
- Contact information for reference sites
- Case studies or implementation success stories
- Willingness to arrange reference site visits or detailed discussions
Get our free ebook on how you can level up your B2B SaaS content marketing
Get our free ebook on how you can level up your B2B SaaS content marketing
Newsletter-Sign Up ELPRO News
ELPRO will use the information provided in this form to keep in touch with you and to send you updates and marketing information by e-mail.








